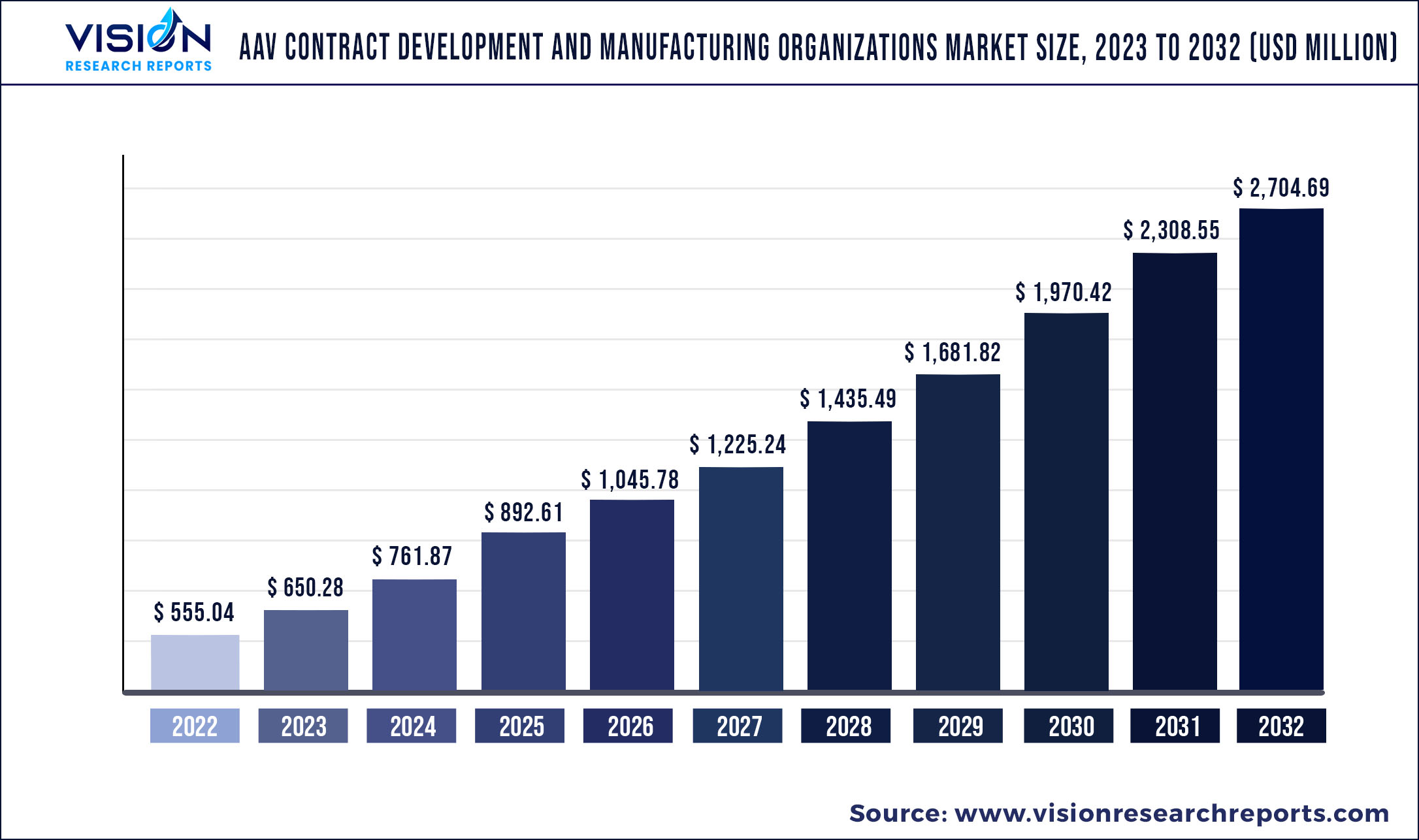
The global AAV contract development and manufacturing organizations market was estimated at USD 555.04 million in 2022 and it is expected to surpass around USD 2,704.69 million by 2032, poised to grow at a CAGR of 17.16% from 2023 to 2032.
Key Pointers
Report Scope of the AAV Contract Development And Manufacturing Organizations Market
| Report Coverage | Details |
| Market Size in 2022 | USD 555.04 million |
| Revenue Forecast by 2032 | USD 2,704.69 million |
| Growth rate from 2023 to 2032 | CAGR of 17.16% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Covered | Thermo Fischer Scientific, Inc.; Creative Biogene, Catalent Inc.; Charles River Laboratories International, Inc.; Danaher (Aldevron); Forge Biologics, Genezen; ViroCell Biologics; Merck KGaA; VIRALGEN; Biovian Oy; Esco Lifesciences (Esco Aster Pte. Ltd.); GenScript ProBio; Porton Advanced Solution Ltd.; Ask Bio; Showa Denko; Takara Bio, Inc.; ABL Manufacturing, Oxford Biomedica; Belief Biomed, Inc.; Beijing Anlong Biomedicine Co. Ltd.; Forecyte Bio Limited; Gene Pharma, Inc.; Skyline Therapeutics; TFBS Bioscience, Inc. |
This growth can be attributed to the active evaluation of adeno-associated viral vectors for the development of various therapies for the treatment of infectious and chronic diseases, the rising number of AAV for vaccines and the rising number of corporate strategies such as expansion, mergers & acquisitions, product launches drive market growth.
The COVID-19 restrictions interrupted research-based operations and the progress of the healthcare industry. However, adeno-associated viral vector contract manufacturing market witnessed growth in 2020 and 2021. High market growth during the COVID-19 pandemic can be attributed to increased research activities seeking efficient treatment for the COVID-19 infection. The urgent need for vaccine research because of the pandemic impelled the market growth. Adeno-associated viral vector vaccines are comparatively easy to produce than live, attenuated, or recombinant vaccinations, as they do not require the same complicated methods. As these vaccines are relatively stable at room temperature, cold storage is not required for their transportation anywhere in the world.
Adeno-Associated Virus (AAV), adenovirus, or lentivirus vectors are currently used in most gene therapies in the market. For instance, in 2021, adeno-associated virus vector was used in more than 250 clinical trials and 82 gene therapy. Gene therapies that are directly administered to patients, by infusion or local administration (in vivo), commonly involve AAV and viral vectors, with AAV being the most preferred vector for applications other than cancer and vaccines.
Improving viral vector manufacturing for gene therapy applications is an important step in developing effective end products. In recent years, the biopharmaceutical industry has witnessed an activity spike, with service providers like Contract Development and Manufacturing Organizations (CDMOs) having invested in expansions of viral vector manufacturing capacity or viral vector development technologies. For instance, in August 2022, Merck KGaA launched its new, UpTempo Virtuos platform process for the CGMP manufacturing and development of adeno-associated viral (AAV) vectors. This platform would streamline & standardize various time-consuming steps in AAV manufacturing to reduce the timeline from gene to clinic and enable rapid first-in-human clinical evaluation.
Regional Insights
North America held the largest revenue share of 50.13% in 2022 due to the presence of established CMOs in the region providing adeno-associated virus manufacturing services, and a growing number of adeno-associated virus vaccines. Also, U.S. FDA is the body that governs the regulatory framework process on adeno-associated viruses in the U.S. region.
Asia Pacific is expected to expand at the fastest rate of 19.72% across the forecast period. This is due to the availability of a significant number of Food and Drug Administration (FDA), Therapeutic Goods Administration (TGA), and European Medicines Agency (EMA) approved facilities in the region that have improved contract manufacturing activities. Also, the low cost of manufacturing in the region is expected to increase the outsourcing of adeno-associated virus vector manufacturing services in the region.
AAV Contract Development And Manufacturing Organizations Market Segmentations:
By Workflow
By Culture Type
By Application
By End-user
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on AAV Contract Development And Manufacturing Organizations Market
5.1. COVID-19 Landscape: AAV Contract Development And Manufacturing Organizations Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global AAV Contract Development And Manufacturing Organizations Market, By Workflow
8.1. AAV Contract Development And Manufacturing Organizations Market, by Workflow, 2023-2032
8.1.1. Upstream Processing
8.1.1.1. Market Revenue and Forecast (2020-2032)
8.1.2. Downstream Processing
8.1.2.1. Market Revenue and Forecast (2020-2032)
Chapter 9. Global AAV Contract Development And Manufacturing Organizations Market, By Culture Type
9.1. AAV Contract Development And Manufacturing Organizations Market, by Culture Type, 2023-2032
9.1.1. Adherent Culture
9.1.1.1. Market Revenue and Forecast (2020-2032)
9.1.2. Suspension Culture
9.1.2.1. Market Revenue and Forecast (2020-2032)
Chapter 10. Global AAV Contract Development And Manufacturing Organizations Market, By Application
10.1. AAV Contract Development And Manufacturing Organizations Market, by Application, 2023-2032
10.1.1. Cell & Gene Therapy Development
10.1.1.1. Market Revenue and Forecast (2020-2032)
10.1.2. Vaccine Development
10.1.2.1. Market Revenue and Forecast (2020-2032)
10.1.3. Biopharmaceutical & Pharmaceutical Discovery
10.1.3.1. Market Revenue and Forecast (2020-2032)
10.1.4. Biomedical Research
10.1.4.1. Market Revenue and Forecast (2020-2032)
Chapter 11. Global AAV Contract Development And Manufacturing Organizations Market, By End-user
11.1. AAV Contract Development And Manufacturing Organizations Market, by End-user, 2023-2032
11.1.1. Pharmaceutical & Biopharmaceutical Companies
11.1.1.1. Market Revenue and Forecast (2020-2032)
11.1.2. Academic & Research Institutes
11.1.2.1. Market Revenue and Forecast (2020-2032)
Chapter 12. Global AAV Contract Development And Manufacturing Organizations Market, Regional Estimates and Trend Forecast
12.1. North America
12.1.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.1.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.1.3. Market Revenue and Forecast, by Application (2020-2032)
12.1.4. Market Revenue and Forecast, by End-user (2020-2032)
12.1.5. U.S.
12.1.5.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.1.5.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.1.5.3. Market Revenue and Forecast, by Application (2020-2032)
12.1.5.4. Market Revenue and Forecast, by End-user (2020-2032)
12.1.6. Rest of North America
12.1.6.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.1.6.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.1.6.3. Market Revenue and Forecast, by Application (2020-2032)
12.1.6.4. Market Revenue and Forecast, by End-user (2020-2032)
12.2. Europe
12.2.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.2.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.2.3. Market Revenue and Forecast, by Application (2020-2032)
12.2.4. Market Revenue and Forecast, by End-user (2020-2032)
12.2.5. UK
12.2.5.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.2.5.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.2.5.3. Market Revenue and Forecast, by Application (2020-2032)
12.2.5.4. Market Revenue and Forecast, by End-user (2020-2032)
12.2.6. Germany
12.2.6.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.2.6.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.2.6.3. Market Revenue and Forecast, by Application (2020-2032)
12.2.6.4. Market Revenue and Forecast, by End-user (2020-2032)
12.2.7. France
12.2.7.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.2.7.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.2.7.3. Market Revenue and Forecast, by Application (2020-2032)
12.2.7.4. Market Revenue and Forecast, by End-user (2020-2032)
12.2.8. Rest of Europe
12.2.8.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.2.8.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.2.8.3. Market Revenue and Forecast, by Application (2020-2032)
12.2.8.4. Market Revenue and Forecast, by End-user (2020-2032)
12.3. APAC
12.3.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.3.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.3.3. Market Revenue and Forecast, by Application (2020-2032)
12.3.4. Market Revenue and Forecast, by End-user (2020-2032)
12.3.5. India
12.3.5.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.3.5.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.3.5.3. Market Revenue and Forecast, by Application (2020-2032)
12.3.5.4. Market Revenue and Forecast, by End-user (2020-2032)
12.3.6. China
12.3.6.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.3.6.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.3.6.3. Market Revenue and Forecast, by Application (2020-2032)
12.3.6.4. Market Revenue and Forecast, by End-user (2020-2032)
12.3.7. Japan
12.3.7.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.3.7.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.3.7.3. Market Revenue and Forecast, by Application (2020-2032)
12.3.7.4. Market Revenue and Forecast, by End-user (2020-2032)
12.3.8. Rest of APAC
12.3.8.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.3.8.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.3.8.3. Market Revenue and Forecast, by Application (2020-2032)
12.3.8.4. Market Revenue and Forecast, by End-user (2020-2032)
12.4. MEA
12.4.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.4.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.4.3. Market Revenue and Forecast, by Application (2020-2032)
12.4.4. Market Revenue and Forecast, by End-user (2020-2032)
12.4.5. GCC
12.4.5.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.4.5.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.4.5.3. Market Revenue and Forecast, by Application (2020-2032)
12.4.5.4. Market Revenue and Forecast, by End-user (2020-2032)
12.4.6. North Africa
12.4.6.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.4.6.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.4.6.3. Market Revenue and Forecast, by Application (2020-2032)
12.4.6.4. Market Revenue and Forecast, by End-user (2020-2032)
12.4.7. South Africa
12.4.7.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.4.7.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.4.7.3. Market Revenue and Forecast, by Application (2020-2032)
12.4.7.4. Market Revenue and Forecast, by End-user (2020-2032)
12.4.8. Rest of MEA
12.4.8.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.4.8.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.4.8.3. Market Revenue and Forecast, by Application (2020-2032)
12.4.8.4. Market Revenue and Forecast, by End-user (2020-2032)
12.5. Latin America
12.5.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.5.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.5.3. Market Revenue and Forecast, by Application (2020-2032)
12.5.4. Market Revenue and Forecast, by End-user (2020-2032)
12.5.5. Brazil
12.5.5.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.5.5.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.5.5.3. Market Revenue and Forecast, by Application (2020-2032)
12.5.5.4. Market Revenue and Forecast, by End-user (2020-2032)
12.5.6. Rest of LATAM
12.5.6.1. Market Revenue and Forecast, by Workflow (2020-2032)
12.5.6.2. Market Revenue and Forecast, by Culture Type (2020-2032)
12.5.6.3. Market Revenue and Forecast, by Application (2020-2032)
12.5.6.4. Market Revenue and Forecast, by End-user (2020-2032)
Chapter 13. Company Profiles
13.1. Thermo Fischer Scientific, Inc.
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. Creative Biogene
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. Catalent Inc.
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. Charles River Laboratories International, Inc.
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. Danaher (Aldevron)
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. Forge Biologics
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. Genezen
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. ViroCell Biologics
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. Merck KGaA
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. VIRALGEN
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others