The global active wound care market was valued at USD 1,077.9 million in 2021 and it is predicted to surpass around USD 2.3 billion by 2030 with a CAGR of 8.79% from 2022 to 2030.
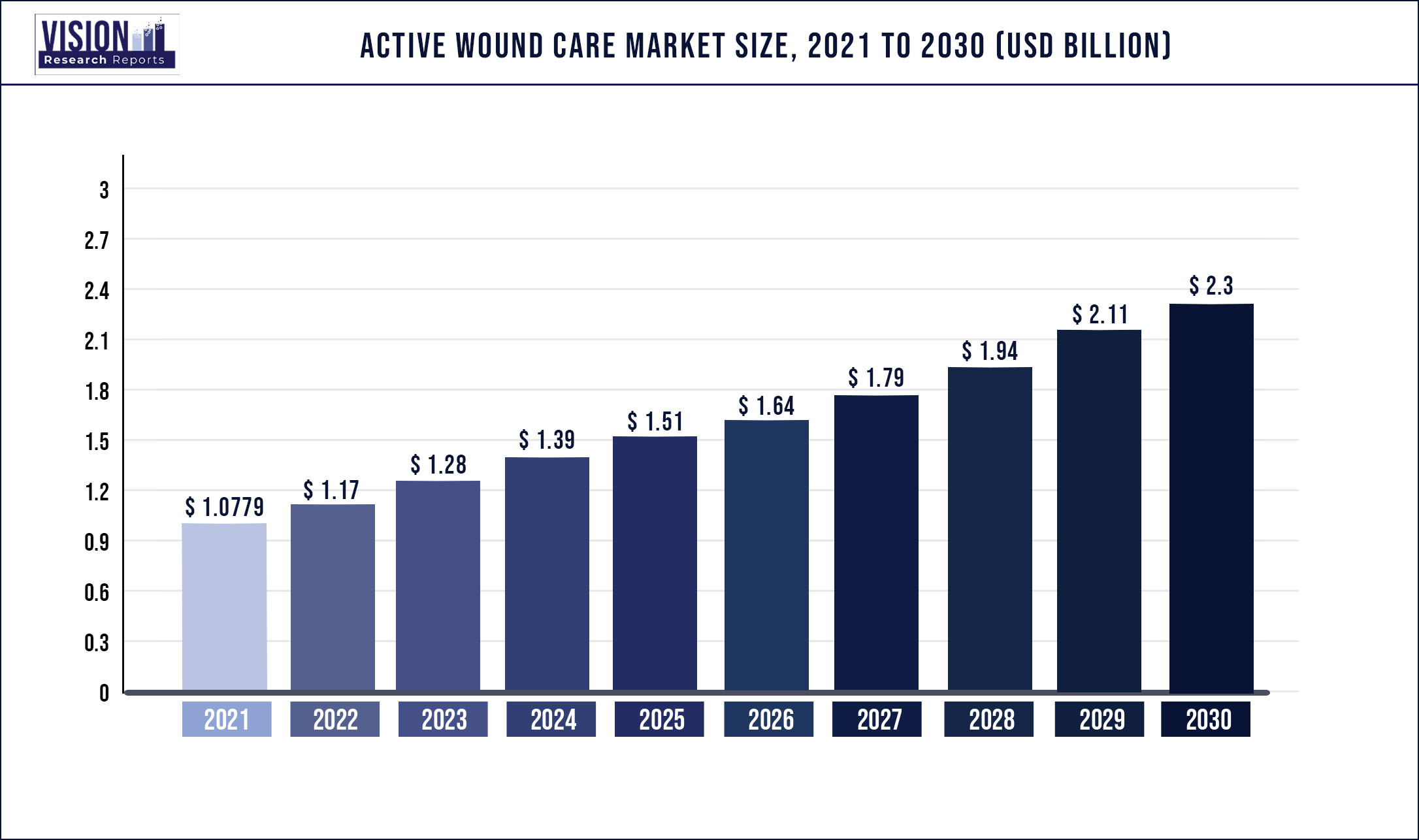
The increasing prevalence of chronic and acute wounds, rapidly aging population, rising number of diabetic patients, and surge in research and development activities are the major factors driving the market.
The rising demand for skin grafts is a primary driver of market expansion. Burn care and treatment have recently changed to a more holistic strategy that focuses not only on healing from burn injury but also on enhancement in long-term function and shape of the healed lesion, as well as the quality of life. As a result of this trend, the need for grafting and other substitutes in the treatment and management of acute burns has increased and continues to rise.
Skin grafts are commonly utilized on partial and full-thickness burns and play a significant role in burn injury treatment. Furthermore, these grafts give a more intact extracellular matrix, which benefits wound healing. Because of the existence of a foundation membrane, skin grafts allow for efficient re-epithelization and provide greater control over scaffold composition. In terms of absorbency and frequency of dressing change, it also outperforms other dressing materials. The aforementioned benefits are expected to fuel the market's overall expansion.
Furthermore, there are several growth factors known to impose significant effects on surgical use, including PDGF, VEGF, FGF, epidermal growth factor (EGF), keratinocyte growth factor (KGF), transforming growth factor-beta (TGF-), granulocyte-macrophage colony-stimulating factor (GM-CSF), and others, due to advancements in genetic engineering and biological technology. Growth factors attract cells into wounds, boost their proliferation, and have a great impact on extracellular matrix deposition. It has led to statistically significant improvements in tissue repair. Such factors are contributing to market growth.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 1,077.9 million |
| Revenue Forecast by 2030 | USD 2.3 billion |
| Growth rate from 2022 to 2030 | CAGR of 8.79% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Product, application, end-use, region |
| Companies Covered | Smith & Nephew; MiMedx; Tissue Regenix; Organogenesis Inc.; Acell Inc.; Integra Life Sciences; Solsys Medical; Osiris Therapeutics Inc.; Cytori Therapeutics Inc.; Human BioSciences; Wright Medical Group N.V |
Product Insights
The biomaterials segment accounted for the maximum share of 44.38% of the global revenue in 2021. The segment growth can be attributed to rapid technological advancements involving the usage of biomaterials for wound healing therapies. These wound care products comprise both synthetic and natural substances that help in both wound healing and tissue regeneration. Moreover, a surge in research and development activities and continual launches by key players is further contributing to the segment growth. For instance, in November 2020, UCLA researchers in collaboration with Duke University, developed a wound-healing biomaterial that could reduce scar formation and allow skin tissue to regenerate, resulting in healthier and stronger skin. Similarly, in February 2019, Axio Biosolutions launched MaxioCel, a next-generation wound care dressing made of chitosan. Thereby such developments are propelling the segment growth.
The skin-substitutes segment is expected to witness the fastest CAGR of 5.39%. Skin substitutes are tissue-engineered products that replace the form and function of the skin, either temporarily or permanently. Advanced skin substitutes are now used generally for deep and chronic wounds as they can prevent infection, limit bodily fluid loss, improve cytokine and growth factor production, reduced inflammatory response and subsequent scarring and protect the healing wound by acting as a covering. Moreover, tissue-engineered skin substitutes are an effective technique to address the scarcity of donor skin grafts. Advancements in technology for the development of skin substitutes such as collagen synthetic bilaminates and tissue culture-derived are among the factors expected to contribute to segment growth.
Moreover, with the advancement of biotechnology and tissue engineering, a wide range of skin substitutes for the treatment of chronic wounds are now available in the market for active wound care. Lately, a Tissue Biology Research Unit (TBRU) at the University of Zurich, Switzerland, and numerous laboratories have developed Dermo-epidermal Skin Substitutes (DESSs) comprising dermal and epidermal skin layers. Such laboratory-grown skin substitutes provide a novel and promising therapeutic alternative for patients with severe, full-thickness skin injuries.
The skin-substitute segment is further categorized into biological and synthetic. Biological substitutes dominated the market; however, the synthetic segment is anticipated to witness the fastest CAGR of 5.9% over the forecast period. The main advantage of biological tissue-created skin substitutes is that the patient's skin is easily available. More extracellular matrix is present in these substitutes, which aids in good re-epithelialization and speedier wound healing. On the other hand, the composition of the scaffold in synthetic tissue-designed skin substitutes may be controlled more precisely. Moreover, advances in technology for the production of tissue-engineered skin substitutes such as collagen-synthetic bilaminates and tissue culture-derived membranes are helping to drive segment expansion.
Application Insights
The chronic wounds segment dominated the market for active wound care and held the largest revenue share of 60.6% in 2021 and is anticipated to witness a considerable growth rate over the forecast period. The broad category of skin substitutes and biomaterials offers the ability to promote chronic wound healing and minimize the medical burden caused by these wounds. The increasing prevalence of diabetic foot ulcers, pressure ulcers, venous leg ulcers, and other chronic wounds, are likely to drive segment growth. For instance, according to ScienceDirect, diabetic foot ulcers may affect more than 25% of the diabetic population and may lead to amputation of the foot in 20% of patients. Moreover, according to American Diabetes Association, in 2018, an estimated 34.2 million people, i.e., 10.5% of the total U.S. population, had diabetes.
In addition, as per the data published in Mary Ann Liebert, Inc., in the U.S., a projected 500,000–600,000 people have venous leg ulcers resulting in an approximately USD 1 billion burden on health care. Thus, with the increasing number of patients suffering from chronic diseases is likely to spur the demand for active wound care products. Furthermore, the rising geriatric population is also a key factor driving the segment growth. According to the Administration for Community Living's profile on older Americans, the U.S. population aged 65 and above is estimated to be 54.1 million in 2019, accounting for approximately 19% of the total U.S. population. From 2009 to 2019, the population expanded by around 36%, and it is expected to reach 80.8 million by 2040, and 94.7 million by 2060. Older people are more susceptible and prone to injury, which may lead to an increase in the incidence of chronic wounds.
The acute wounds segment is expected to witness a considerable CAGR of 4.1% during the forecast period. The increasing cases of different traumatic wounds and burns are the major factor driving the segment. For instance, around 50.0% of people worldwide are exposed to fire-related traumas and among them, 90.0% of the cases occur in low to moderate-income countries. Similarly, as per the WHO, over 1,000,000 people are registered, annually, as moderately or severely burnt in India. Moreover, according to a study published by ScienceDirect in 2017, approximately 195,000 deaths occur in Indonesia annually due to burns. Such a rising prevalence of burn injuries is expected to create high demand for skin substitutes, thereby driving the segment growth.
End-use Insights
The hospital segment dominated the market for active wound care and held the largest revenue share of 45.2% in 2021, as a higher number of patients prefer to visit hospitals for different cases such as surgical wounds, burns, and ulcers. Moreover, the increasing number of surgical procedures and an increasing number of hospitals are among the major factors driving the segment. For instance, as per the latest survey by the American Hospital Association, the total number of hospitals in the U.S. was counted to be 6,093 in 2022. Hence, owing to the aforementioned factors, the segment is anticipated to propel during the forecast period.
The home healthcare segment is expected to witness the fastest CAGR of 5.4% during the forecast period. Homecare is expected to witness the fastest growth over the forecast period. The onset of the COVID-19 pandemic mainly led to the promotion of home healthcare. Moreover, the geriatric population prefers homecare over hospital stay. Various conditions such as diabetic foot ulcers, venous leg ulcers, and surgical wounds generally require prolonged hospital stays, which often becomes challenging for elderly patients.
Furthermore, owing to the increasing healthcare costs, a large number of patients suffering from chronic diseases prefer to undergo treatments in-home care settings. This trend is likely to surge the demand for active wound care products, as they are used by patients to treat, diagnose, and monitor a variety of chronic and acute wounds in-home care settings. Hence, such factors are anticipated to boost segment growth over the forecast period.
Regional Insights
North America dominated the active wound care market and accounted for a revenue share of 45.2% in 2021 and is expected to witness a considerable growth rate over the forecast period. There is a rising demand for advanced treatment options, owing to the increasing prevalence of wounds, rising economic cost burden, and growing efforts by the government to reduce the overall treatment duration. These factors have led to the growing number of regulatory approvals for active wound care products including skin substitutes and growth factors in the U.S. This, along with suitable reimbursement policies in the region, are factors responsible for regional dominance.
In the Asia Pacific, the market for active wound care is estimated to witness the highest CAGR of 5.3% over the forecast period. This can be attributed to rising changing lifestyles leading to an increase in the incidence of chronic diseases and the presence of a large population base. For instance, in 2017, Diabetic Foot Ulcers (DFUs) account for roughly 80% of all non-traumatic amputations in India, according to a research paper published in Value in Health (the official journal of the International Society for Pharmacoeconomics and Outcomes Research, Inc.). Furthermore, patients with a history of DFU had a 40% higher 10-year mortality rate than those without. Such developments are projected to boost demand for active wound care products as an increasing number of patients uses these products to enhance patient treatment results. Furthermore, medical tourism in this region is increasing which is increasing the number of surgeries performed. Additionally, the increasing focus of the major players in the emerging Asian countries and government support is further driving the growth of the market for active wound care in this region.
Key Players
Market Segmentation
Chapter 1. Introduction
1.1.Research Objective
1.2.Scope of the Study
1.3.Definition
Chapter 2. Research Methodology
2.1.Research Approach
2.2.Data Sources
2.3.Assumptions & Limitations
Chapter 3. Executive Summary
3.1.Market Snapshot
Chapter 4. Market Variables and Scope
4.1.Introduction
4.2.Market Classification and Scope
4.3.Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and End-use Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Active Wound Care Market
5.1.COVID-19 Landscape: Active Wound Care Industry Impact
5.2.COVID 19 - Impact Assessment for the Industry
5.3.COVID 19 Impact: Global Major Government Policy
5.4.Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1.Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2.Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Active Wound Care Market, By Product
8.1.Active Wound Care Market, by Product Type, 2022-2030
8.1.1. Biomaterials
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Skin-substitutes
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Growth Factors
8.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Active Wound Care Market, By Application
9.1.Active Wound Care Market, by Application, 2022-2030
9.1.1. Chronic Wounds
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Acute Wounds
9.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 10.Global Active Wound Care Market, By End-use
10.1.Active Wound Care Market, by End-use, 2022-2030
10.1.1.Hospitals
10.1.1.1.Market Revenue and Forecast (2017-2030)
10.1.2.Specialty Clinics
10.1.2.1.Market Revenue and Forecast (2017-2030)
10.1.3.Home Healthcare
10.1.3.1.Market Revenue and Forecast (2017-2030)
10.1.4.Others
10.1.4.1.Market Revenue and Forecast (2017-2030)
Chapter 11.Global Active Wound Care Market, Regional Estimates and Trend Forecast
11.1.North America
11.1.1.Market Revenue and Forecast, by Product (2017-2030)
11.1.2.Market Revenue and Forecast, by Application (2017-2030)
11.1.3.Market Revenue and Forecast, by End-use (2017-2030)
11.1.4.U.S.
11.1.4.1.Market Revenue and Forecast, by Product (2017-2030)
11.1.4.2.Market Revenue and Forecast, by Application (2017-2030)
11.1.4.3.Market Revenue and Forecast, by End-use (2017-2030)
11.1.5.Rest of North America
11.1.5.1.Market Revenue and Forecast, by Product (2017-2030)
11.1.5.2.Market Revenue and Forecast, by Application (2017-2030)
11.1.5.3.Market Revenue and Forecast, by End-use (2017-2030)
11.2.Europe
11.2.1.Market Revenue and Forecast, by Product (2017-2030)
11.2.2.Market Revenue and Forecast, by Application (2017-2030)
11.2.3.Market Revenue and Forecast, by End-use (2017-2030)
11.2.4.UK
11.2.4.1.Market Revenue and Forecast, by Product (2017-2030)
11.2.4.2.Market Revenue and Forecast, by Application (2017-2030)
11.2.4.3.Market Revenue and Forecast, by End-use (2017-2030)
11.2.5.Germany
11.2.5.1.Market Revenue and Forecast, by Product (2017-2030)
11.2.5.2.Market Revenue and Forecast, by Application (2017-2030)
11.2.5.3.Market Revenue and Forecast, by End-use (2017-2030)
11.2.6.France
11.2.6.1.Market Revenue and Forecast, by Product (2017-2030)
11.2.6.2.Market Revenue and Forecast, by Application (2017-2030)
11.2.6.3.Market Revenue and Forecast, by End-use (2017-2030)
11.2.7.Rest of Europe
11.2.7.1.Market Revenue and Forecast, by Product (2017-2030)
11.2.7.2.Market Revenue and Forecast, by Application (2017-2030)
11.2.7.3.Market Revenue and Forecast, by End-use (2017-2030)
11.3.APAC
11.3.1.Market Revenue and Forecast, by Product (2017-2030)
11.3.2.Market Revenue and Forecast, by Application (2017-2030)
11.3.3.Market Revenue and Forecast, by End-use (2017-2030)
11.3.4.India
11.3.4.1.Market Revenue and Forecast, by Product (2017-2030)
11.3.4.2.Market Revenue and Forecast, by Application (2017-2030)
11.3.4.3.Market Revenue and Forecast, by End-use (2017-2030)
11.3.5.China
11.3.5.1.Market Revenue and Forecast, by Product (2017-2030)
11.3.5.2.Market Revenue and Forecast, by Application (2017-2030)
11.3.5.3.Market Revenue and Forecast, by End-use (2017-2030)
11.3.6.Japan
11.3.6.1.Market Revenue and Forecast, by Product (2017-2030)
11.3.6.2.Market Revenue and Forecast, by Application (2017-2030)
11.3.6.3.Market Revenue and Forecast, by End-use (2017-2030)
11.3.7.Rest of APAC
11.3.7.1.Market Revenue and Forecast, by Product (2017-2030)
11.3.7.2.Market Revenue and Forecast, by Application (2017-2030)
11.3.7.3.Market Revenue and Forecast, by End-use (2017-2030)
11.4.MEA
11.4.1.Market Revenue and Forecast, by Product (2017-2030)
11.4.2.Market Revenue and Forecast, by Application (2017-2030)
11.4.3.Market Revenue and Forecast, by End-use (2017-2030)
11.4.4.GCC
11.4.4.1.Market Revenue and Forecast, by Product (2017-2030)
11.4.4.2.Market Revenue and Forecast, by Application (2017-2030)
11.4.4.3.Market Revenue and Forecast, by End-use (2017-2030)
11.4.5.North Africa
11.4.5.1.Market Revenue and Forecast, by Product (2017-2030)
11.4.5.2.Market Revenue and Forecast, by Application (2017-2030)
11.4.5.3.Market Revenue and Forecast, by End-use (2017-2030)
11.4.6.South Africa
11.4.6.1.Market Revenue and Forecast, by Product (2017-2030)
11.4.6.2.Market Revenue and Forecast, by Application (2017-2030)
11.4.6.3.Market Revenue and Forecast, by End-use (2017-2030)
11.4.7.Rest of MEA
11.4.7.1.Market Revenue and Forecast, by Product (2017-2030)
11.4.7.2.Market Revenue and Forecast, by Application (2017-2030)
11.4.7.3.Market Revenue and Forecast, by End-use (2017-2030)
11.5.Latin America
11.5.1.Market Revenue and Forecast, by Product (2017-2030)
11.5.2.Market Revenue and Forecast, by Application (2017-2030)
11.5.3.Market Revenue and Forecast, by End-use (2017-2030)
11.5.4.Brazil
11.5.4.1.Market Revenue and Forecast, by Product (2017-2030)
11.5.4.2.Market Revenue and Forecast, by Application (2017-2030)
11.5.4.3.Market Revenue and Forecast, by End-use (2017-2030)
11.5.5.Rest of LATAM
11.5.5.1.Market Revenue and Forecast, by Product (2017-2030)
11.5.5.2.Market Revenue and Forecast, by Application (2017-2030)
11.5.5.3.Market Revenue and Forecast, by End-use (2017-2030)
Chapter 12.Company Profiles
12.1.Smith & Nephew
12.1.1.Company Overview
12.1.2.Product Offerings
12.1.3.Financial Performance
12.1.4.Recent Initiatives
12.2.MiMedx
12.2.1.Company Overview
12.2.2.Product Offerings
12.2.3.Financial Performance
12.2.4.Recent Initiatives
12.3.Tissue Regenix
12.3.1.Company Overview
12.3.2.Product Offerings
12.3.3.Financial Performance
12.3.4.Recent Initiatives
12.4.Organogenesis Inc.
12.4.1.Company Overview
12.4.2.Product Offerings
12.4.3.Financial Performance
12.4.4.Recent Initiatives
12.5.Acell Inc.
12.5.1.Company Overview
12.5.2.Product Offerings
12.5.3.Financial Performance
12.5.4.Recent Initiatives
12.6.Integra Life Sciences
12.6.1.Company Overview
12.6.2.Product Offerings
12.6.3.Financial Performance
12.6.4.Recent Initiatives
12.7.Solsys Medical
12.7.1.Company Overview
12.7.2.Product Offerings
12.7.3.Financial Performance
12.7.4.Recent Initiatives
12.8.Osiris Therapeutics Inc.
12.8.1.Company Overview
12.8.2.Product Offerings
12.8.3.Financial Performance
12.8.4.Recent Initiatives
12.9.Cytori Therapeutics Inc.
12.9.1.Company Overview
12.9.2.Product Offerings
12.9.3.Financial Performance
12.9.4.Recent Initiatives
12.10.Human BioSciences
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
12.11.Wright Medical Group N.V.
12.11.1. Company Overview
12.11.2. Product Offerings
12.11.3. Financial Performance
12.11.4. Recent Initiative
Chapter 13.Research Methodology
13.1.Primary Research
13.2.Secondary Research
13.3.Assumptions
Chapter 14.Appendix
14.1.About Us
14.2.Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others