The Canada latent tuberculosis infection detection market was estimated at USD 27.4 million in 2021 and it is expected to surpass around USD 47.31 million by 2030, poised to grow at a CAGR of 6.26% from 2022 to 2030.
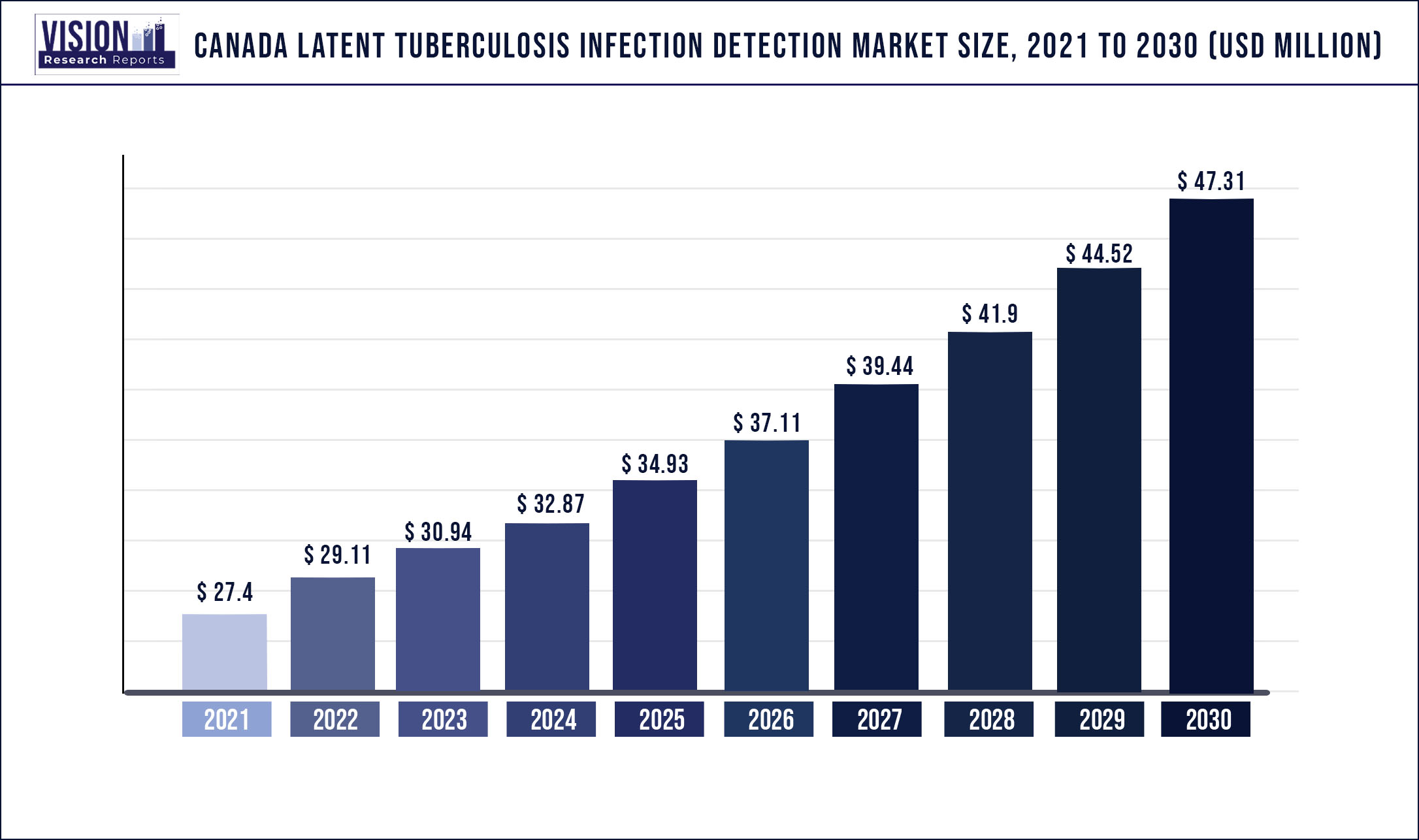
The rising infection rate of latent TB in the indigenous population and high inbound traveling from the regions where TB is endemic are the major factors increasing the demand for latent tuberculosis infection screening. Moreover, the surge in tuberculosis testing and ongoing research activities to develop novel TB tests is another factor contributing to market expansion.
The increasing incidence of latent tuberculosis infection in the country is expected to fuel the demand for testing. Most people infected with M. tuberculosis remain asymptomatic, however, about 5% of latent TB infections develop into active cases. The goal of public health authorities to test for LTBI and to identify people at high risk of developing TB disease is increasing the demand for tuberculosis testing.
According to data published by the Canadian Medical Association, around 70% of immigrants to Canada come from Asia, Africa, and Latin American countries where TB infection is endemic. Out of these immigrants, around 50% are estimated to have latent tuberculosis infection. Moreover, around two-thirds of reported cases occur in foreign-born individuals in Canada.
Canada’s government is taking favorable initiatives to achieve WHO’s goal to eliminate the disease in the country. It has already achieved the End TB target milestone, an action plan by the WHO to eliminate the disease by 2050. Optimal management of tuberculosis and improvement in healthcare infrastructure is expected to eradicate the disease in the coming years.
Moreover, increasing recommendations for latent TB infection tests for the high-risk population in the country is expected to fuel market growth. The Canadian TB Standards recommend the use of TST and IGRA for the detection of LTBI. IGRAs are preferred to test LTBI in individuals who have taken the BCG vaccine and in groups with poor TST test results. However, TST is mainly recommended when repeated testing is planned.
The screening for LTBI of immigrants and the general population in the country is low. About 2.5% of new immigrants are flagged for surveillance after reaching the country. Medical screening programs for immigrants in Canada do not specifically include screening for LTBI and it is not mentioned in most lists of reportable diseases in the country. The inclusion of LTBI screening in surveillance programs in the country may increase the demand for latent tuberculosis tests in the coming years.
The reimbursement for tests used to detect latent tuberculosis infection is provided for people who have been identified as having contact with active cases or those at high risk of developing active TB. The reimbursement for TB testing is decentralized in the country and is provided at the province level with certain criteria. The government does not recommend latent tuberculosis infection testing and reimbursement to the general population in the country.
Most states in Canada have established three priority strategies for the prevention and management of the disease; identification of people with tuberculosis infection, investigation of contacts of infectious TB, and investigation of the population at high risk for latent TB infection. Such strategies to manage the disease in the country will increase the demand for screening tests for tuberculosis infections.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 27.4 million |
| Revenue Forecast by 2030 | USD 47.31 million |
| Growth rate from 2022 to 2030 | CAGR of 6.26% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Test type, end-use, province |
| Companies Covered |
F. Hoffmann-La Roche Ltd, Abbott, QIAGEN, BD (Becton, Dickinson, and Company), bioMérieux SA., Oxford Immunotec USA, Inc. |
Test Type Insights
Based on test type, the interferon-gamma release assay segment was the major revenue generator in 2021. Increasing awareness about TB infection and increasing recommendations for IGRAs from various public health authorities has boosted its adoption for LTBI detection. Health Canada has approved two IGRAs tests; QuantiFERON-TB Gold (QFT-GIT) assay by Qiagen and T-SPOT.TB assay by Oxford Immunotec for the detection of latent TB infections.
The IGRA segment is anticipated to experience the fastest growth over the forecast period. High test sensitivity and increasing adoption of IGRAs due to their advantages over TSTs are some of the key factors driving the segment growth over the forecast period.
However, the tuberculin skin test segment dominated in terms of the number of tests performed for the detection of LTBI. High market penetration and cost-effectiveness of the test have boosted the adoption of tests in the country. In Canada, Tubersol 5 tuberculin units of purified protein derivative are recommended to conduct a tuberculin skin test. Sanofi is one of the major players providing tuberculin-purified protein derivatives in Canada.
End-use Insights
The diagnostic laboratories segment dominated the Canada latent tuberculosis infection detection market owing to efforts to improve patient outcomes by providing precise & prompt reporting at the retail level. Moreover, the ability of laboratories to handle a large volume of tests at an expedited rate is expected to further fuel the segment over the forecast period. Diagnostics laboratories such as Dynacare and Life Labs provide blood tests for the detection of latent TB infection in the country.
The hospital & clinics segment held the second-largest market share in 2021 owing to the high risk of getting tuberculosis infections in healthcare facilities. Moreover, individuals with medical conditions, such as HIV/AIDS, chronic renal failure, and other diseases that weaken the immune system, increase the risk of LTBI and its development into an active TB case. However, the academic & research institutions segment is expected to experience the fastest growth rate over the forecast period.
Regional Insights
Canada’s latent tuberculosis infection detection industry is classified into provinces and territories. Ontario province led the market and it is expected to maintain its dominance over the forecast period. The large market share of the province can be attributed to its large population base, high immigration rate compared to other provinces, and better healthcare infrastructure. The immigration rate is highest in Ontario; it welcomes around 40% of the total immigrants of Canada every year. Similarly, British Columbia, Quebec, Alberta, and Manitoba also held a significant share of the LTBI detection market.
North Canada region including Yukon, Northwest Territories, and Nunavut is expected to grow at a faster growth rate compared with other parts of Canada. Improvement of healthcare facilities, a high portion of the indigenous population, and other factors increasing the risk of developing tuberculosis disease are driving the growth of the market in these regions.
Key Players
Market Segmentation
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Canada Latent Tuberculosis Infection Detection Market
5.1. COVID-19 Landscape: Canada Latent Tuberculosis Infection Detection Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Canada Latent Tuberculosis Infection Detection Market, By Test Type
8.1. Canada Latent Tuberculosis Infection Detection Market, by Test Type, 2022-2030
8.1.1 Tuberculin Skin Test
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Interferon Gamma Release Assays
8.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Canada Latent Tuberculosis Infection Detection Market, By End-use
9.1. Canada Latent Tuberculosis Infection Detection Market, by End-use, 2022-2030
9.1.1. Diagnostic Laboratories
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Hospitals/Clinics
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Academic & Research Institutions
9.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Canada Latent Tuberculosis Infection Detection Market, By Province
10.1. Canada Latent Tuberculosis Infection Detection Market, by Province, 2022-2030
10.1.1. Alberta
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. British Columbia
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Manitoba
10.1.3.1. Market Revenue and Forecast (2017-2030)
10.1.4. New Brunswick
10.1.4.1. Market Revenue and Forecast (2017-2030)
10.1.5. Nova Scotia
10.1.5.1. Market Revenue and Forecast (2017-2030)
10.1.6. Ontario
10.1.6.1. Market Revenue and Forecast (2017-2030)
10.1.7. Prince Edward Island
10.1.7.1. Market Revenue and Forecast (2017-2030)
10.1.8. Quebec
10.1.8.1. Market Revenue and Forecast (2017-2030)
10.1.9. Saskatchewan
10.1.9.1. Market Revenue and Forecast (2017-2030)
10.1.10. Northwest Territories
10.1.10.1. Market Revenue and Forecast (2017-2030)
10.1.11. Nunavut
10.1.11.1. Market Revenue and Forecast (2017-2030)
10.1.12. Yukon
10.1.12.1. Market Revenue and Forecast (2017-2030)
10.1.13. Newfoundland & Labrador
10.1.13.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Canada Latent Tuberculosis Infection Detection Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.1.2. Market Revenue and Forecast, by End-use (2017-2030)
11.1.3. Market Revenue and Forecast, by Province (2017-2030)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.1.4.2. Market Revenue and Forecast, by End-use (2017-2030)
11.1.4.3. Market Revenue and Forecast, by Province (2017-2030)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.1.5.2. Market Revenue and Forecast, by End-use (2017-2030)
11.1.5.3. Market Revenue and Forecast, by Province (2017-2030)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.2.2. Market Revenue and Forecast, by End-use (2017-2030)
11.2.3. Market Revenue and Forecast, by Province (2017-2030)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.2.4.2. Market Revenue and Forecast, by End-use (2017-2030)
11.2.4.3. Market Revenue and Forecast, by Province (2017-2030)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.2.5.2. Market Revenue and Forecast, by End-use (2017-2030)
11.2.5.3. Market Revenue and Forecast, by Province (2017-2030)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.2.6.2. Market Revenue and Forecast, by End-use (2017-2030)
11.2.6.3. Market Revenue and Forecast, by Province (2017-2030)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.2.7.2. Market Revenue and Forecast, by End-use (2017-2030)
11.2.7.3. Market Revenue and Forecast, by Province (2017-2030)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.3.2. Market Revenue and Forecast, by End-use (2017-2030)
11.3.3. Market Revenue and Forecast, by Province (2017-2030)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.3.4.2. Market Revenue and Forecast, by End-use (2017-2030)
11.3.4.3. Market Revenue and Forecast, by Province (2017-2030)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.3.5.2. Market Revenue and Forecast, by End-use (2017-2030)
11.3.5.3. Market Revenue and Forecast, by Province (2017-2030)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.3.6.2. Market Revenue and Forecast, by End-use (2017-2030)
11.3.6.3. Market Revenue and Forecast, by Province (2017-2030)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.3.7.2. Market Revenue and Forecast, by End-use (2017-2030)
11.3.7.3. Market Revenue and Forecast, by Province (2017-2030)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.4.2. Market Revenue and Forecast, by End-use (2017-2030)
11.4.3. Market Revenue and Forecast, by Province (2017-2030)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.4.4.2. Market Revenue and Forecast, by End-use (2017-2030)
11.4.4.3. Market Revenue and Forecast, by Province (2017-2030)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.4.5.2. Market Revenue and Forecast, by End-use (2017-2030)
11.4.5.3. Market Revenue and Forecast, by Province (2017-2030)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.4.6.2. Market Revenue and Forecast, by End-use (2017-2030)
11.4.6.3. Market Revenue and Forecast, by Province (2017-2030)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.4.7.2. Market Revenue and Forecast, by End-use (2017-2030)
11.4.7.3. Market Revenue and Forecast, by Province (2017-2030)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.5.2. Market Revenue and Forecast, by End-use (2017-2030)
11.5.3. Market Revenue and Forecast, by Province (2017-2030)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.5.4.2. Market Revenue and Forecast, by End-use (2017-2030)
11.5.4.3. Market Revenue and Forecast, by Province (2017-2030)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Test Type (2017-2030)
11.5.5.2. Market Revenue and Forecast, by End-use (2017-2030)
11.5.5.3. Market Revenue and Forecast, by Province (2017-2030)
Chapter 12. Company Profiles
12.1. Abbott
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. QIAGEN
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. F. Hoffmann-La Roche Ltd
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Sanofi
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Oxford Immunotec USA
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. ARKRAY, Inc.
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. bioMérieux SA
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others