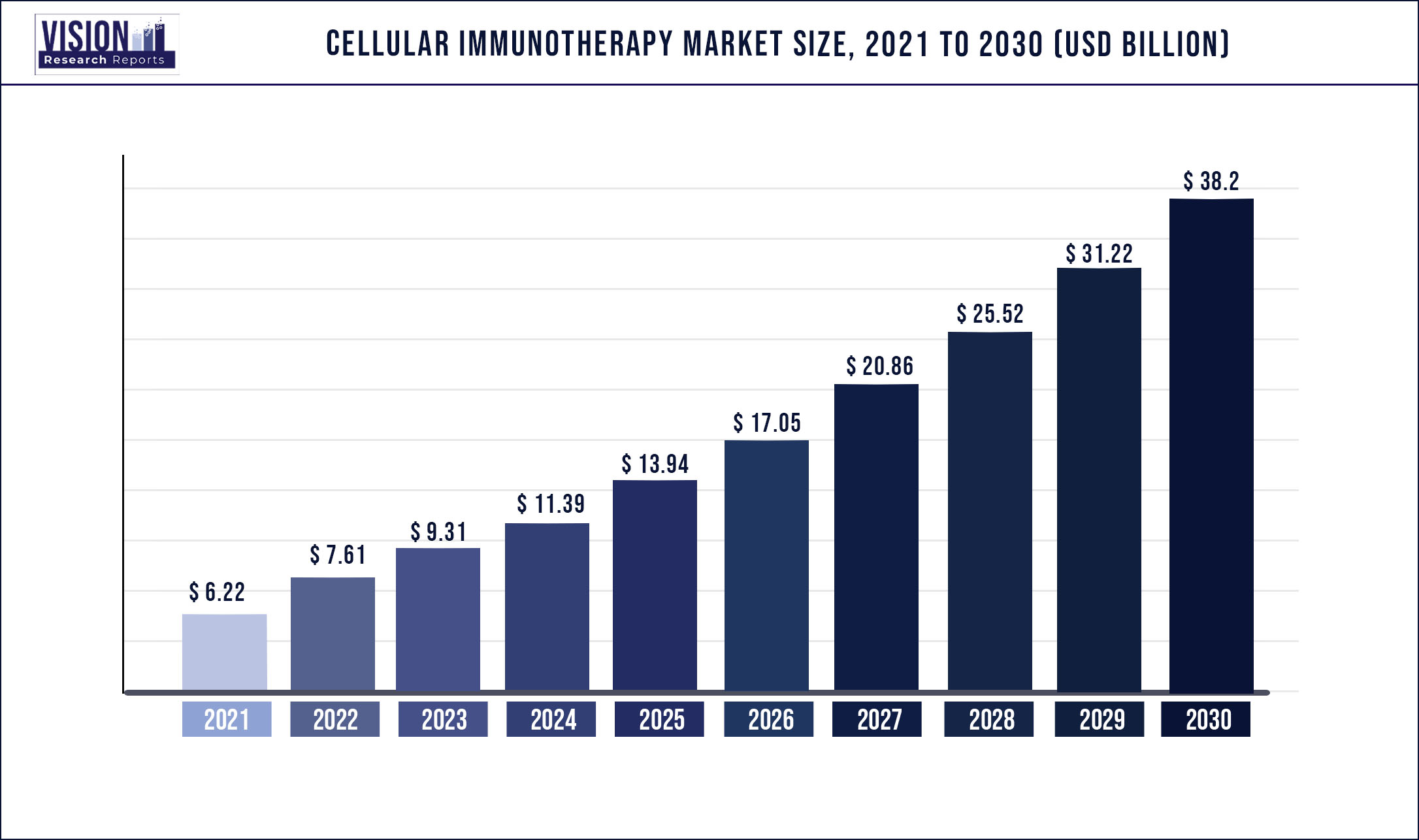
The global cellular immunotherapy market was surpassed at USD 6.22 billion in 2021 and is expected to hit around USD 38.2 billion by 2030, growing at a CAGR of 22.34% from 2022 to 2030.
Report Highlights
Growing support from government organizations and research institutes including the National Cancer Institute (NCI) and Center of Excellence in Immunology (CEI) for cellular immunotherapies research is one of the major factors driving the market growth. For instance, the CEI pulls out experts from other institutes such as NCI and NIH to promote the growth of immunotherapy for the treatment of cancer. Also, the Surgery Branch of the National Cancer Institute's Center for Cancer Research (CCR) is committed to the innovation of novel immunotherapies for the treatment of cancer patients.
The rising number of M&As, collaborations, acquisitions, and funding is a major trend observed in the market. M&A and collaboration help companies expand their existing product portfolio and regional reach in a short period. For instance, in March 2021, TrakCel received funding from Labcorp and AmerisourceBergen for cellular therapy orchestration research and global expansion. Also, in April 2020, Kite (a Gilead Company) and Teneobio, Inc. entered into a license agreement through which Kite will receive exclusive rights to specific antibodies targeted to B-cell maturation antigen (BCMA). Thus, such M&A and collaborations are expected to boost the market growth.
A substantial number of cellular immunotherapy companies are engaged in new product launches, pertaining to licensing, collaborations, & acquisitions, and regional expansion. Some of the key companies in the market are Bristol-Myers Squibb Company; Novartis AG; Gilead Sciences Inc. (Kite Pharma); F. Hoffmann-La Roche Ltd.; and Johnson & Johnson. These companies are adopting various business models to gain a competitive advantage. For instance, in February 2021, Novartis secured approval for the first Australian commercial CAR-T manufacturing site for Kymriah. With this approval, the company would be able to develop Kymriah in Australia, thereby providing easy access to Australian patients.
The COVID-19 pandemic has had a high impact on the market. Owing to stringent regulations to curb the pandemic, a slowdown in clinical trials, approval of new drugs, and delayed cancer diagnosis have been observed, affecting the industry's growth amid the pandemic. However, in several countries, the effect of COVID-19 declined after the third quarter of 2021, which helped the market regain traction.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 6.22 billion |
| Revenue Forecast by 2030 | USD 38.2 billion |
| Growth rate from 2022 to 2030 | CAGR of 22.34% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Therapy type, indication, end-use, region |
| Companies Covered | Bristol-Myers Squibb Company; Novartis AG; Gilead Sciences Inc. (Kite Pharma); F. Hoffmann-La Roche Ltd; Merck KGaA; GlaxoSmithKline plc.; AstraZeneca; Pfizer Inc.; Johnson & Johnson; Celyad, Adicet Bio, Inc.; Dendreon Pharmaceuticals LLC |
Therapy Type Insights
CAR T cell therapy held the largest market share of the market and is expected to expand at the fastest rate during the forecast period. This can be attributed to a lucrative product pipeline and new product approvals. Owing to its promising therapeutic results, market players are extensively invested in the R&D of new CAR T cell therapy. Currently, there are more than 500 trials underway for CAR T cell therapy. New product approvals and subsequent launches of products are expected to accelerate the market growth during the forthcoming years. For instance, in February 2022, Janssen Global Services, LLC’s CARVYKTI (ciltacabtagene autoleucel) received FDA approval. It is indicated for the treatment of multiple myeloma.
However, high cost of therapy and improper reimbursement scenario, especially in lower-income and middle-income countries, are anticipated to impede the growth. For instance, the one-time infusion cost for Cilta-cel is around 465,000 USD.
Indication Insights
The B-cell malignancy segment accounted for the highest revenue share in 2021 and is expected to advance at the fastest rate throughout the forecast period, which can be attributed to high cellular immunotherapy product penetration for the treatment of B-cell malignancy. Availability of products such as BREYANZI and Yescarta for treating B-cell malignancy are among the primary factors contributing to its high market growth. BREYANZI received FDA approval in February 2021; in its first financial year, it generated a revenue of approximately USD 87 million.
Prostate cancer is expected to hold a significant market share of cellular immunotherapy in 2021. It is the second most commonly occurring cancer in the world after lung cancer. According to WHO, in 2020, it was estimated that 1.4 million people were diagnosed with prostate cancer globally, and this number is expected to grow in the coming decades. In addition, the availability of advanced products such as Sipuleucel-T is another factor that supports the segment growth.
End-use Insights
Hospitals dominated the end-use segment in the global market in 2021. The rising prevalence of cancer has increased the total number of hospitalizations worldwide, leading to a rise in patients in hospital settings. Moreover, infusion of cellular immunotherapy requires skilled healthcare practitioners; such requirements are more compatible with hospital settings, which further increases patient compliance.
Cancer institutes segment is expected to progress at the fastest CAGR during the forecast period. Better availability of products and ease of reimbursement are the major factors propelling the growth of this segment. Cancer institutes offer advanced treatment and care to a high number of patients as well as enable quick reimbursement, resulting in a rising number of patients opting for cancer institute settings.
Regional Insights
North America dominated the market in terms of revenue in 2021, owing to its well-established healthcare infrastructure, availability of advanced therapies, high disposable income, and high per capita healthcare expenditure. The presence of a robust research infrastructure, coupled with a large number of clinical studies being conducted by cell therapy manufacturers in the U.S., has majorly contributed to the dominance of the market in the region.
The Asia Pacific region is expected to exhibit the highest growth rate due to untapped opportunities in the region and growing awareness about cellular immunotherapy, coupled with the rapidly developing healthcare infrastructure. In the Asia Pacific, China is advancing at the fastest CAGR owing to the high number of clinical trials relating to these therapies. Also, growing government investment and favorable regulations in China have resulted in a lucrative growth rate in the region.
Key Players
Market Segmentation
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Cellular Immunotherapy Market
5.1. COVID-19 Landscape: Cellular Immunotherapy Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Cellular Immunotherapy Market, By Therapy Type
8.1. Cellular Immunotherapy Market, by Therapy Type, 2022-2030
8.1.1 CAR T Cell Therapy
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Dendritic Cell Therapy
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. NK Cell Therapy
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. TIL Therapy
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Others
8.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Cellular Immunotherapy Market, By Indication
9.1. Cellular Immunotherapy Market, by Indication, 2022-2030
9.1.1. B-cell Malignancies
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Prostate Cancer
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Liver Cancer
9.1.3.1. Market Revenue and Forecast (2017-2030)
9.1.4. Renal Cell Carcinoma
9.1.4.1. Market Revenue and Forecast (2017-2030)
9.1.5. Others
9.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Cellular Immunotherapy Market, By End-use
10.1. Cellular Immunotherapy Market, by End-use, 2022-2030
10.1.1. Hospitals
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Cancer Institutes
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Others
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Cellular Immunotherapy Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.1.2. Market Revenue and Forecast, by Indication (2017-2030)
11.1.3. Market Revenue and Forecast, by End-use (2017-2030)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.1.4.2. Market Revenue and Forecast, by Indication (2017-2030)
11.1.4.3. Market Revenue and Forecast, by End-use (2017-2030)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.1.5.2. Market Revenue and Forecast, by Indication (2017-2030)
11.1.5.3. Market Revenue and Forecast, by End-use (2017-2030)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.2.2. Market Revenue and Forecast, by Indication (2017-2030)
11.2.3. Market Revenue and Forecast, by End-use (2017-2030)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.2.4.2. Market Revenue and Forecast, by Indication (2017-2030)
11.2.4.3. Market Revenue and Forecast, by End-use (2017-2030)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.2.5.2. Market Revenue and Forecast, by Indication (2017-2030)
11.2.5.3. Market Revenue and Forecast, by End-use (2017-2030)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.2.6.2. Market Revenue and Forecast, by Indication (2017-2030)
11.2.6.3. Market Revenue and Forecast, by End-use (2017-2030)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.2.7.2. Market Revenue and Forecast, by Indication (2017-2030)
11.2.7.3. Market Revenue and Forecast, by End-use (2017-2030)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.3.2. Market Revenue and Forecast, by Indication (2017-2030)
11.3.3. Market Revenue and Forecast, by End-use (2017-2030)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.3.4.2. Market Revenue and Forecast, by Indication (2017-2030)
11.3.4.3. Market Revenue and Forecast, by End-use (2017-2030)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.3.5.2. Market Revenue and Forecast, by Indication (2017-2030)
11.3.5.3. Market Revenue and Forecast, by End-use (2017-2030)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.3.6.2. Market Revenue and Forecast, by Indication (2017-2030)
11.3.6.3. Market Revenue and Forecast, by End-use (2017-2030)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.3.7.2. Market Revenue and Forecast, by Indication (2017-2030)
11.3.7.3. Market Revenue and Forecast, by End-use (2017-2030)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.4.2. Market Revenue and Forecast, by Indication (2017-2030)
11.4.3. Market Revenue and Forecast, by End-use (2017-2030)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.4.4.2. Market Revenue and Forecast, by Indication (2017-2030)
11.4.4.3. Market Revenue and Forecast, by End-use (2017-2030)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.4.5.2. Market Revenue and Forecast, by Indication (2017-2030)
11.4.5.3. Market Revenue and Forecast, by End-use (2017-2030)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.4.6.2. Market Revenue and Forecast, by Indication (2017-2030)
11.4.6.3. Market Revenue and Forecast, by End-use (2017-2030)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.4.7.2. Market Revenue and Forecast, by Indication (2017-2030)
11.4.7.3. Market Revenue and Forecast, by End-use (2017-2030)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.5.2. Market Revenue and Forecast, by Indication (2017-2030)
11.5.3. Market Revenue and Forecast, by End-use (2017-2030)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.5.4.2. Market Revenue and Forecast, by Indication (2017-2030)
11.5.4.3. Market Revenue and Forecast, by End-use (2017-2030)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Therapy Type (2017-2030)
11.5.5.2. Market Revenue and Forecast, by Indication (2017-2030)
11.5.5.3. Market Revenue and Forecast, by End-use (2017-2030)
Chapter 12. Company Profiles
12.1. CAR T Cell Therapy
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Dendritic Cell Therapy
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. NK Cell Therapy
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. TIL Therapy
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Others
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others