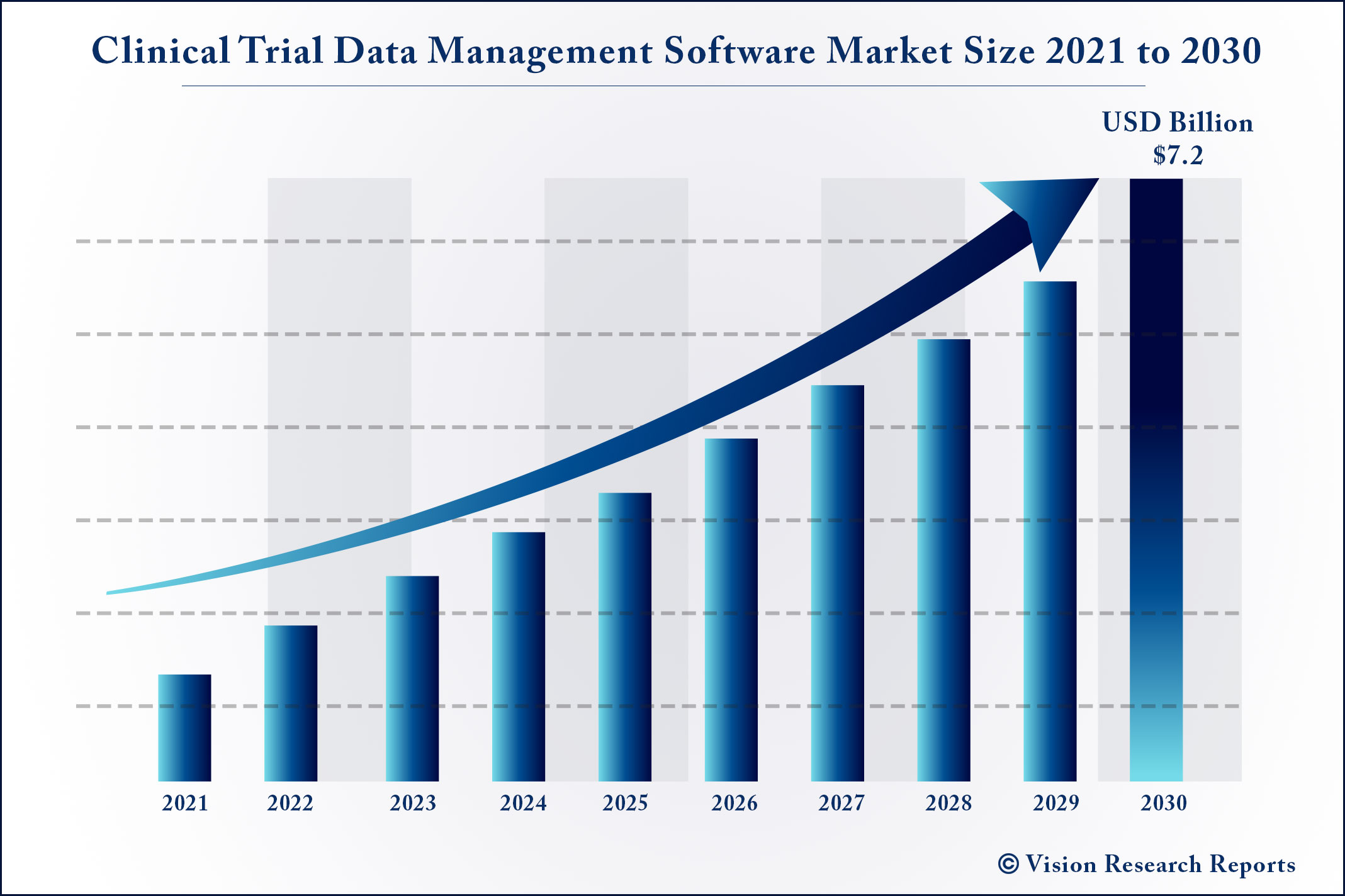
The global clinical trial data management software market size is expected to surpass around USD 7.2 billion by 2030 and is anticipated to grow at a CAGR of 8.1% from 2021 to 2030.
Clinical trial data management is the process of handling data generated during the clinical trials of drugs, vaccines, etc. The main use of clinical trial data management software is to manage, produce, and maintain quality data involved in a clinical trial, including planning, preparation, performance, and reporting. In this report, we analyze the challenges end users face in acquiring the right clinical trial data management software that can cope with the rapidly changing world. The report also highlights the total revenue generated through the sale of clinical trial data management software across various key regions worldwide.
The global clinical trial data management software market is currently driven by growing demand from pharmaceutical & biomedical companies to reduce operational costs and failures of clinical trials
Use of Digital Technologies in Clinical Research Driving Global Market
Technology plays a critical role in drug development. Rising innovation in technologies enables end users to adopt technologically advanced clinical trial data management software. End users are currently facing some challenges such as failure during patient enrollment, long and delayed trial processes, and low clinical trial success rate. Demand for clinical trial data management software is increasing in pharmaceutical and biotech companies, and medical device companies to overcome these challenges. Increasing spending by pharmaceutical companies on Artificial Intelligence, Blockchain, and Big Data analytics are the key factors boosting huge investments in clinical trial data management software by end users.
Increase in clinical research outsourcing
Outsourcing of clinical trial activities in different regions of the world is a key driver of the clinical trial data management software market. For instance, in Asia Pacific, outsourcing of clinical trials has increased due to the rapid growth of the pharmaceutical market, which in turn is projected to fuel the demand for clinical trial data management software during the forecast period.
Key Challenges
Report Highlights
Based on component, the clinical trial data management software market has been classified into software and services. Software segment dominated the global clinical trial data management software market in 2020 and is expected to maintain its dominance during the forecast period. The software segment has been sub-classified into on-premises/enterprise and on demand Software as a Service (SaaS). Services has been sub-classified into professional and managed.
Based on end user, the clinical trial data management software market has been categorized into pharmaceutical & biotech companies, medical device companies, and third party/contract research organizations. The pharmaceutical & biotech companies segment is expected to account for leading share of the global clinical trial data management software market. The segment is projected to continue its dominance in the coming years, as it is realizing the urgent need to monitor & record the data of patients and reduce operational cost.
In terms of region, the North America is expected to dominate the clinical trial data management software market during the forecast period. The U.S. is expected to lead the North America clinical trial data management software market. According to several clinical trial databases such ClinicalTrials.gov, the European Organisation for Research and Treatment of Cancer, and International Clinical Trials Registry Platform, the number of clinical trials performed in the U.S. is almost six times more than in Canada.
The clinical trial data management software market in Asia Pacific is projected to expand at a high CAGR during the forecast period. Adoption of cloud-based clinical trial data management software by end users has propelled the demand for clinical trial data management software in the region.
Key Players
Key players profiled in the report include Bioclinica, Bio-Optronics, Forte Research Systems, IBM Corporation, Medidata Solutions, Oracle Corporation, Parexel, Quad One Technologies Pvt. Ltd., Trial By Fire Solutions, and Veeva Systems Inc.
Market Segmentation
Market By Component
Market By End User
Market By Region
This report focuses on clinical trial data management software market includes crucial information on market share, market size, and growth rate for the forecast period 2021 to 2030 at the global level, regional level and company level. From a global perspective, this report represents overall clinical trial data management software market size by analyzing historical data and future prospect. The study highlights deep analysis on the major drivers of the market, restraints, and challenges to help the business owners, suppliers, and marketing personnel in planning effective strategies for the forecast period. This will help the business and manufacturers to lead the market and gain prominent position in future. The report also presents vital information through graphical representation on factors like table, charts, and statistics. The study includes drivers and restraints of the global clinical trial data management software market.
The research not only conducts forecasts in terms of value, but also evaluates the market on the basis of essential parameters, such as Year-on-Year (Y-o-Y) growth. This helps providers to recognize the future opportunities as well predictability of the market.
In order to understand and assess opportunities in this market, the report is categorically divided into five key sections on the basis of segments. The report analyzes the global market in terms of value (US$ dollers) and volume (Million Units).
The research report includes specific segments by region (country), by company, by all segments. This study provides information about the growth and revenue during the historic and forecasted period of 2017 to 2030. Every segment is further sub-segmented into several sub-segmented that are deeply analyzed by experts to offer valuable information to the buyers and market players. Understanding the segments helps in identifying the importance of different factors that aid the market growth.
Major Key Points Covered in Report:
Executive Summary: It includes key trends of the clinical trial data management software market related to products, applications, and other crucial factors. It also provides analysis of the competitive landscape and CAGR and market size of the clinical trial data management software market based on production and revenue.
Production and Consumption by Region: It covers all regional markets to which the research study relates. Prices and key players in addition to production and consumption in each regional market are discussed.
Key Players: Here, the report throws light on financial ratios, pricing structure, production cost, gross profit, sales volume, revenue, and gross margin of leading and prominent companies competing in the Clinical trial data management software market.
Market Segments: This part of the report discusses about product, application and other segments of the clinical trial data management software market based on market share, CAGR, market size, and various other factors.
Research Methodology: This section discusses about the research methodology and approach used to prepare the report. It covers data triangulation, market breakdown, market size estimation, and research design and/or programs.
Regional Analysis
The research report includes a detailed study of regions of North America, Europe, China, Japan and Rest of the World. The report has been curated after observing and studying various factors that determine regional growth such as economic, environmental, social, technological, and political status of the particular region. Analysts have studied the data of revenue and manufacturers of each region. This section analyses region-wise revenue and volume for the forecast period of 2017 to 2030. These analyses will help the reader to understand the potential worth of investment in a particular region.
The report provides in-depth segment analysis of the global clinical trial data management software market, thereby providing valuable insights at macro as well as micro levels. Analysis of major countries, which hold growth opportunities or account for significant share has also been included as part of geographic analysis of the clinical trial data management software market.
The report includes country-wise and region-wise market size for the period 2017-2030. It also includes market size and forecast by segments in terms of production capacity, price and revenue for the period 2017-2030.
Competitive Landscape and Market Share Analysis
The clinical trial data management software market competitive landscape provides details by vendors, including company overview, company total revenue (financials), market potential, global presence, clinical trial data management software sales and revenue generated, market share, price, production sites and facilities, SWOT analysis, product launch. For the period 2017-2020, this study provides the clinical trial data management software sales, revenue and market share for each player covered in this report.
Research Methodology
The research methodology adopted by analysts for compiling the global clinical trial data management software market report is based on detailed primary as well as secondary research. With the help of in-depth insights of the market-affiliated information that is obtained and legitimated by market-admissible resources, analysts have offered riveting observations and authentic forecasts for the global market.
During the primary research phase, analysts interviewed market stakeholders, investors, brand managers, vice presidents, and sales and marketing managers. Based on data obtained through interviews of genuine resources, analysts have emphasized the changing scenario of the global market.
For secondary research, analysts scrutinized numerous annual report publications, white papers, market association publications, and company websites to obtain the necessary understanding of the global clinical trial data management software market.
The study objectives of this report are:
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Clinical Trial Data Management Software Market, By Component
7.1. Clinical Trial Data Management Software Market, by Component, 2021-2030
7.1.1. Software
7.1.1.1. Market Revenue and Forecast (2016-2030)
7.1.2. Services
7.1.2.1. Market Revenue and Forecast (2016-2030)
Chapter 8. Global Clinical Trial Data Management Software Market, By End User
8.1. Clinical Trial Data Management Software Market, by End User, 2021-2030
8.1.1. Pharmaceutical & Biotech Companies
8.1.1.1. Market Revenue and Forecast (2016-2030)
8.1.2. Medical Device Companies
8.1.2.1. Market Revenue and Forecast (2016-2030)
8.1.3. Third Party/Contract Research Organizations
8.1.3.1. Market Revenue and Forecast (2016-2030)
Chapter 9. Global Clinical Trial Data Management Software Market, Regional Estimates and Trend Forecast
9.1. North America
9.1.1. Market Revenue and Forecast, by Component (2016-2030)
9.1.2. Market Revenue and Forecast, by End User (2016-2030)
9.1.3. U.S.
9.1.3.1. Market Revenue and Forecast, by Component (2016-2030)
9.1.3.2. Market Revenue and Forecast, by End User (2016-2030)
9.1.4. Rest of North America
9.1.4.1. Market Revenue and Forecast, by Component (2016-2030)
9.1.4.2. Market Revenue and Forecast, by End User (2016-2030)
9.2. Europe
9.2.1. Market Revenue and Forecast, by Component (2016-2030)
9.2.2. Market Revenue and Forecast, by End User (2016-2030)
9.2.3. UK
9.2.3.1. Market Revenue and Forecast, by Component (2016-2030)
9.2.3.2. Market Revenue and Forecast, by End User (2016-2030)
9.2.4. Germany
9.2.4.1. Market Revenue and Forecast, by Component (2016-2030)
9.2.4.2. Market Revenue and Forecast, by End User (2016-2030)
9.2.5. France
9.2.5.1. Market Revenue and Forecast, by Component (2016-2030)
9.2.5.2. Market Revenue and Forecast, by End User (2016-2030)
9.2.6. Rest of Europe
9.2.6.1. Market Revenue and Forecast, by Component (2016-2030)
9.2.6.2. Market Revenue and Forecast, by End User (2016-2030)
9.3. APAC
9.3.1. Market Revenue and Forecast, by Component (2016-2030)
9.3.2. Market Revenue and Forecast, by End User (2016-2030)
9.3.3. India
9.3.3.1. Market Revenue and Forecast, by Component (2016-2030)
9.3.3.2. Market Revenue and Forecast, by End User (2016-2030)
9.3.4. China
9.3.4.1. Market Revenue and Forecast, by Component (2016-2030)
9.3.4.2. Market Revenue and Forecast, by End User (2016-2030)
9.3.5. Japan
9.3.5.1. Market Revenue and Forecast, by Component (2016-2030)
9.3.5.2. Market Revenue and Forecast, by End User (2016-2030)
9.3.6. Rest of APAC
9.3.6.1. Market Revenue and Forecast, by Component (2016-2030)
9.3.6.2. Market Revenue and Forecast, by End User (2016-2030)
9.4. MEA
9.4.1. Market Revenue and Forecast, by Component (2016-2030)
9.4.2. Market Revenue and Forecast, by End User (2016-2030)
9.4.3. GCC
9.4.3.1. Market Revenue and Forecast, by Component (2016-2030)
9.4.3.2. Market Revenue and Forecast, by End User (2016-2030)
9.4.4. North Africa
9.4.4.1. Market Revenue and Forecast, by Component (2016-2030)
9.4.4.2. Market Revenue and Forecast, by End User (2016-2030)
9.4.5. South Africa
9.4.5.1. Market Revenue and Forecast, by Component (2016-2030)
9.4.5.2. Market Revenue and Forecast, by End User (2016-2030)
9.4.6. Rest of MEA
9.4.6.1. Market Revenue and Forecast, by Component (2016-2030)
9.4.6.2. Market Revenue and Forecast, by End User (2016-2030)
9.5. Latin America
9.5.1. Market Revenue and Forecast, by Component (2016-2030)
9.5.2. Market Revenue and Forecast, by End User (2016-2030)
9.5.3. Brazil
9.5.3.1. Market Revenue and Forecast, by Component (2016-2030)
9.5.3.2. Market Revenue and Forecast, by End User (2016-2030)
9.5.4. Rest of LATAM
9.5.4.1. Market Revenue and Forecast, by Component (2016-2030)
9.5.4.2. Market Revenue and Forecast, by End User (2016-2030)
Chapter 10. Company Profiles
10.1. Bioclinica
10.1.1. Company Overview
10.1.2. Product Offerings
10.1.3. Financial Performance
10.1.4. Recent Initiatives
10.2. Bio-Optronics
10.2.1. Company Overview
10.2.2. Product Offerings
10.2.3. Financial Performance
10.2.4. Recent Initiatives
10.3. Forte Research Systems
10.3.1. Company Overview
10.3.2. Product Offerings
10.3.3. Financial Performance
10.3.4. Recent Initiatives
10.4. IBM Corporation
10.4.1. Company Overview
10.4.2. Product Offerings
10.4.3. Financial Performance
10.4.4. Recent Initiatives
10.5. Medidata Solutions
10.5.1. Company Overview
10.5.2. Product Offerings
10.5.3. Financial Performance
10.5.4. Recent Initiatives
10.6. Oracle Corporation
10.6.1. Company Overview
10.6.2. Product Offerings
10.6.3. Financial Performance
10.6.4. Recent Initiatives
10.7. Parexel
10.7.1. Company Overview
10.7.2. Product Offerings
10.7.3. Financial Performance
10.7.4. Recent Initiatives
10.8. Quad One Technologies Pvt. Ltd.
10.8.1. Company Overview
10.8.2. Product Offerings
10.8.3. Financial Performance
10.8.4. Recent Initiatives
10.9. Trial By Fire Solutions
10.9.1. Company Overview
10.9.2. Product Offerings
10.9.3. Financial Performance
10.9.4. Recent Initiatives
10.10. Veeva Systems Inc.
10.10.1. Company Overview
10.10.2. Product Offerings
10.10.3. Financial Performance
10.10.4. Recent Initiatives
Chapter 11. Research Methodology
11.1. Primary Research
11.2. Secondary Research
11.3. Assumptions
Chapter 12. Appendix
12.1. About Us
12.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others