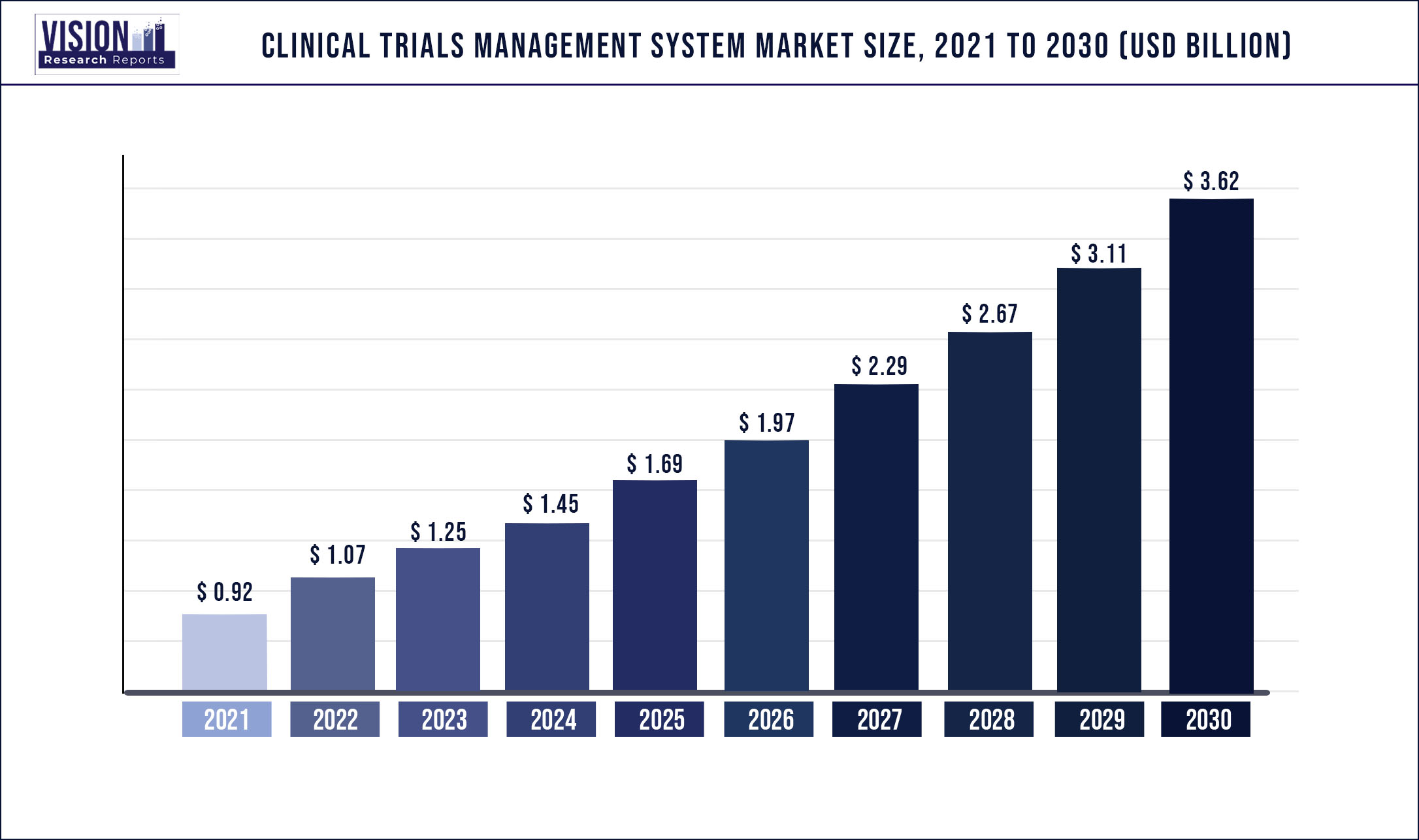
The global Clinical Trials Management System market size is expected to be worth around US$ 3.62 billion by 2030, according to a new report by Vision Research Reports.
The global Clinical Trials Management System market size was valued at US$ 792.07 million in 2020 and is anticipated to grow at a CAGR of 16.1% during forecast period 2021 to 2030.
Growth Factors
Rapid growth of healthcare IT, high R&D expenditure by life science and clinical research organizations, and adoption of CTMS solutions is anticipated to drive the market growth.
Increasing investment by pharmaceutical and biotechnology companies coupled with government funding is promoting research activities. This factor is expected to boostgrowth of the market for clinical trial management systems.According to the estimates of the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), global research-based pharmaceutical industry has spent around USD 149.8 billion in 2015. The annual spending of pharmaceutical industry is 5.5 times greater than the aerospace industry, 1.8 times more than software and computer services andsoftware industry, and 5 times more than chemicals industry.
Report Highlights
Based on type, the market for clinical trials management systems is segmented into enterprise and site. Enterprise CTMS is leading the market due to benefits such as real-time insights into the operational activities such as accruals and deviations, robust reporting, enhanced billing compliance, and tracking and management of regulatory processes. In addition, enterprise CTMS provides stronger support for financial management and helps bring consistency in the budget. Oracle Corporation; Forte Research Systems Inc.; and Bio-Optronics, Inc. are some of the companies that offer enterprise CTMS.
Web-based systems held the largest share in 2016 owing to benefits such as remote access to data and minimal technical issues. Web-based systems are the most preferred CTM systems. They help minimize cost associated with system security, backups, upgrades, and uptime consistency. Furthermore, web-based software allows the centralization of data, which facilitates access to data from any location. Web-based CTMS uses secure enterprise-class data centers to store, update, and back-up on a daily basis; minimizing technical concerns for users. These factors are expected to drive growth during the forecast period.
On the other hand, a cloud-based system is anticipated to exhibit the fastest growth over the forecast period. These systems have successfully outgrown web-based systems owing to constantly evolving applications. This technology comprises three services such as Platform as a Service (PaaS), Infrastructure as a Service (IaaS), and Software as a Service (SaaS). Cloud-based technology offers greater study control to CTMS, wherein the clinical trial managers can manage location and other research study requirements in real-time. Another advantage is access to data from any device such as mobile, workstations, laptops, and tablets through software.
In addition, cloud-based CTMS eliminates the expenses of hardware acquisition, installation, provisioning, maintenance, support, and software licensing. These systems automatically update software and patch management systems, which reduces the burden of in-house IT staff and saves costs. Furthermore, the cloud-based software enables access to the server through mobile with maximum data security.
Based on components, the market is segmented into software and services. The software segment held a substantial market share in 2016 and is expected to expand at a steady CAGR over the forecast period. The software performs critical functions such as comprehensive management of trial planning, country and site progress, monitoring activities, and supplies and finance. Periodic software upgrades are necessary for synchronization with latest analytics.
Based on end-use, the market is segmented into pharmaceutical and biotechnology firms, Contract Research Organization (CROs), and medical device firms. The pharmaceutical and biotechnology firms led the market in 2016.CTMS plays a vital role in drug discovery through FDA approval and in developing a new medicine.
Pharmaceutical and biotechnological firms segment occupy the largest revenue share in the CTMS market. This growth can be attributed to rising use in clinical trials and drug development studies by giant pharmaceutical firms. CTMS results in process-driven approach for pharmaceutical companies, which is focused on delivering flexibility and affordability throughout the course of trial development.
Increasing adoption of CTMS can also be attributed to various advantages such as recording of performance matrix, obtaining financial disclosure and medical license, budgeting, and management of documents. However, the market for CROs would grow at the fastest CAGR during the forecast period.
Asia Pacific is expected to be the fastest-growing regional segment during the forecast period. Increasing development cost and time spent on clinical trials and outsourcing of clinical trials has become a viable option. Asian countries offer a less expensive and less time-consuming process for clinical research studies. This factor is anticipated to boost the regional market over the forecast period.
Key Players
Forte Research Systems Inc.; Bioclinica; Oracle Corporation; Medidata Solutions Inc.; DATATRAK; Medpace Holdings, Inc.; Clinical Data Inc.; BioClinica; G.Tech Medical Engineering GmbH; bio-Optronics, Inc.; and PARAXEL International Corporation.
Market Segmentation
Type Outlook
Enterprise
Site
Delivery Mode Outlook
Web-based
Cloud-based
On-premise
Component Outlook
Software
Service
End-User Outlook
Pharmaceutical and Biotechnology Firms
Johnson & Johnson
Roche
Novartis
Pfizer
Merck
GlaxoSmithKline
Novo Nordisk
Amgen
Bristol Myesr Squibb
Sanofi
CROs
Medical Device Firms
Regional Outlook
North America
The U.S.
Canada
Europe
Germany
U.K.
France
Italy
Spain
Russia
Belgium
Netherlands
Asia Pacific
Japan
China
India
Thailand
Australia
Singapore
Malaysia
Indonesia
South Korea
Philippines
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
UAE
Saudi Arabia
South Africa
The Clinical Trials Management System market research report covers definition, classification, product classification, product application, development trend, product technology, competitive landscape, industrial chain structure, industry overview, national policy and planning analysis of the industry, the latest dynamic analysis, etc., and also includes major. The study includes drivers and restraints of the global market. It covers the impact of these drivers and restraints on the demand during the forecast period. The report also highlights opportunities in the market at the global level.
The report provides size (in terms of volume and value) of Clinical Trials Management System market for the base year 2020 and the forecast between 2021 and 2030. Market numbers have been estimated based on form and application. Market size and forecast for each application segment have been provided for the global and regional market.
This report focuses on the global Clinical Trials Management System market status, future forecast, growth opportunity, key market and key players. The study objectives are to present the Clinical Trials Management System market development in United States, Europe and China.
It is pertinent to consider that in a volatile global economy, we haven’t just conducted Clinical Trials Management System market forecasts in terms of CAGR, but also studied the market based on key parameters, including Year-on-Year (Y-o-Y) growth, to comprehend the certainty of the market and to find and present the lucrative opportunities in market.
In terms of production side, this report researches the Clinical Trials Management System capacity, production, value, ex-factory price, growth rate, market share for major manufacturers, regions (or countries) and type.
In terms of consumption side, this report focuses on the consumption of Clinical Trials Management System by regions (countries) and application.
Buyers of the report will have access to verified market figures, including global market size in terms of revenue and volume. As part of production analysis, the authors of the report have provided reliable estimations and calculations for global revenue and volume by Type segment of the global Clinical Trials Management System market. These figures have been provided in terms of both revenue and volume for the period 2017 to 2030. Additionally, the report provides accurate figures for production by region in terms of revenue as well as volume for the same period. The report also includes production capacity statistics for the same period.
With regard to production bases and technologies, the research in this report covers the production time, base distribution, technical parameters, research and development trends, technology sources, and sources of raw materials of major Clinical Trials Management System market companies.
Regarding the analysis of the industry chain, the research of this report covers the raw materials and equipment of Clinical Trials Management System market upstream, downstream customers, marketing channels, industry development trends and investment strategy recommendations. The more specific analysis also includes the main application areas of market and consumption, major regions and Consumption, major Chinese producers, distributors, raw material suppliers, equipment providers and their contact information, industry chain relationship analysis.
The research in this report also includes product parameters, production process, cost structure, and data information classified by region, technology and application. Finally, the paper model new project SWOT analysis and investment feasibility study of the case model.
Overall, this is an in-depth research report specifically for the Clinical Trials Management System industry. The research center uses an objective and fair way to conduct an in-depth analysis of the development trend of the industry, providing support and evidence for customer competition analysis, development planning, and investment decision-making. In the course of operation, the project has received support and assistance from technicians and marketing personnel in various links of the industry chain.
The Clinical Trials Management System market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies’ focus related to Clinical Trials Management System market.
Prominent players in the market are predicted to face tough competition from the new entrants. However, some of the key players are targeting to acquire the startup companies in order to maintain their dominance in the global market. For a detailed analysis of key companies, their strengths, weaknesses, threats, and opportunities are measured in the report by using industry-standard tools such as the SWOT analysis. Regional coverage of key companies is covered in the report to measure their dominance. Key manufacturers of Clinical Trials Management System market are focusing on introducing new products to meet the needs of the patrons. The feasibility of new products is also measured by using industry-standard tools.
Key companies are increasing their investments in research and development activities for the discovery of new products. There has also been a rise in the government funding for the introduction of new Clinical Trials Management System market. These factors have benefited the growth of the global market for Clinical Trials Management System. Going forward, key companies are predicted to benefit from the new product launches and the adoption of technological advancements. Technical advancements have benefited many industries and the global industry is not an exception.
New product launches and the expansion of already existing business are predicted to benefit the key players in maintaining their dominance in the global market for Clinical Trials Management System. The global market is segmented on the basis of region, application, en-users and product type. Based on region, the market is divided into North America, Europe, Asia-Pacific, Latin America and Middle East and Africa (MEA).
In this study, the years considered to estimate the market size of Clinical Trials Management System are as follows:
Reasons to Purchase this Report:
- Market segmentation analysis including qualitative and quantitative research incorporating the impact of economic and policy aspects
- Regional and country level analysis integrating the demand and supply forces that are influencing the growth of the market.
- Market value USD Million and volume Units Million data for each segment and sub-segment
- Competitive landscape involving the market share of major players, along with the new projects and strategies adopted by players in the past five years
- Comprehensive company profiles covering the product offerings, key financial information, recent developments, SWOT analysis, and strategies employed by the major market players
Research Methodology:
In-depth interviews and discussions were conducted with several key market participants and opinion leaders to compile the research report.
This research study involved the extensive usage of both primary and secondary data sources. The research process involved the study of various factors affecting the industry, including the government policy, market environment, competitive landscape, historical data, present trends in the market, technological innovation, upcoming technologies and the technical progress in related industry, and market risks, opportunities, market barriers and challenges. The following illustrative figure shows the market research methodology applied in this report.
Market Size Estimation
Top-down and bottom-up approaches are used to estimate and validate the global market size for company, regional division, product type and application (end users).
The market estimations in this report are based on the selling price (excluding any discounts provided by the manufacturer, distributor, wholesaler or traders). Market share analysis, assigned to each of the segments and regions are achieved through product utilization rate and average selling price.
Major manufacturers & their revenues, percentage splits, market shares, growth rates and breakdowns of the product markets are determined through secondary sources and verified through the primary sources.
All possible factors that influence the markets included in this research study have been accounted for, viewed in extensive detail, verified through primary research, and analyzed to get the final quantitative and qualitative data. The market size for top-level markets and sub-segments is normalized, and the effect of inflation, economic downturns, and regulatory & policy changes or others factors are accounted for in the market forecast. This data is combined and added with detailed inputs and analysis from Vision Research Reports and presented in this report.
Market Breakdown and Data Triangulation
After complete market engineering with calculations for market statistics; market size estimations; market forecasting; market breakdown; and data triangulation. Extensive primary research was conducted to gather information and verify and validate the critical numbers arrived at. In the complete market engineering process, both top-down and bottom-up approaches were extensively used, along with several data triangulation methods, to perform market estimation and market forecasting for the overall market segments and sub-segments listed in this report.
Secondary Sources
Secondary Sources occupies approximately 25% of data sources, such as press releases, annual reports, Non-Profit organizations, industry associations, governmental agencies and customs data, and so on. This research study includes secondary sources; directories; databases such as Bloomberg Business, Wind Info, Hoovers, Factiva (Dow Jones & Company), TRADING ECONOMICS, and avention; Investing News Network; statista; Federal Reserve Economic Data; annual reports; investor presentations; and SEC filings of companies.
Primary Sources
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include product manufacturers (and their competitors), opinion leaders, industry experts, research institutions, distributors, dealer and traders, as well as the raw materials suppliers and producers, etc.
The primary sources from the demand side include industry experts such as business leaders, marketing and sales directors, technology and innovation directors, supply chain executive, end users (product buyers), and related key executives from various key companies and organizations operating in the global market.
The study objectives of this report are:
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Clinical Trials Management System Market, By Type
7.1. Clinical Trials Management System Market, By Type, 2020-2028
7.1.1. Enterprise
7.1.1.1. Market Revenue and Forecast (2016-2028)
7.1.2. Site
7.1.2.1. Market Revenue and Forecast (2016-2028)
Chapter 8. Global Clinical Trials Management System Market, By Delivery Mode
8.1. Clinical Trials Management System Market, By Delivery Mode, 2020-2028
8.1.1. Web-based
8.1.1.1. Market Revenue and Forecast (2016-2028)
8.1.2. Cloud-based
8.1.2.1. Market Revenue and Forecast (2016-2028)
8.1.3. On-premise
8.1.3.1. Market Revenue and Forecast (2016-2028)
Chapter 9. Global Clinical Trials Management System Market, By End User
9.1. Clinical Trials Management System Market, by End User, 2020-2028
9.1.1. Pharmaceutical and Biotechnology Firms
9.1.1.1. Market Revenue and Forecast (2016-2028)
9.1.2. CROs
9.1.2.1. Market Revenue and Forecast (2016-2028)
9.1.3. Medical Device Firms
9.1.3.1. Market Revenue and Forecast (2016-2028)
Chapter 10. Global Clinical Trials Management System Market, By Component
10.1. Clinical Trials Management System Market, By Component, 2020-2028
10.1.1. Software
10.1.1.1. Market Revenue and Forecast (2016-2028)
10.1.2. Service
10.1.2.1. Market Revenue and Forecast (2016-2028)
Chapter 11. Global Clinical Trials Management System Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, By Type (2016-2028)
11.1.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.1.3. Market Revenue and Forecast, by End User (2016-2028)
11.1.4. Market Revenue and Forecast, By Component (2016-2028)
11.1.5. U.S.
11.1.5.1. Market Revenue and Forecast, By Type (2016-2028)
11.1.5.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.1.5.3. Market Revenue and Forecast, by End User (2016-2028)
11.1.5.4. Market Revenue and Forecast, By Component (2016-2028)
11.1.6. Rest of North America
11.1.6.1. Market Revenue and Forecast, By Type (2016-2028)
11.1.6.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.1.6.3. Market Revenue and Forecast, by End User (2016-2028)
11.1.6.4. Market Revenue and Forecast, By Component (2016-2028)
11.2. Europe
11.2.1. Market Revenue and Forecast, By Type (2016-2028)
11.2.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.2.3. Market Revenue and Forecast, by End User (2016-2028)
11.2.4. Market Revenue and Forecast, By Component (2016-2028)
11.2.5. UK
11.2.5.1. Market Revenue and Forecast, By Type (2016-2028)
11.2.5.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.2.5.3. Market Revenue and Forecast, by End User (2016-2028)
11.2.5.4. Market Revenue and Forecast, By Component (2016-2028)
11.2.6. Germany
11.2.6.1. Market Revenue and Forecast, By Type (2016-2028)
11.2.6.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.2.6.3. Market Revenue and Forecast, by End User (2016-2028)
11.2.6.4. Market Revenue and Forecast, By Component (2016-2028)
11.2.7. France
11.2.7.1. Market Revenue and Forecast, By Type (2016-2028)
11.2.7.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.2.7.3. Market Revenue and Forecast, by End User (2016-2028)
11.2.7.4. Market Revenue and Forecast, By Component (2016-2028)
11.2.8. Rest of Europe
11.2.8.1. Market Revenue and Forecast, By Type (2016-2028)
11.2.8.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.2.8.3. Market Revenue and Forecast, by End User (2016-2028)
11.2.8.4. Market Revenue and Forecast, By Component (2016-2028)
11.3. APAC
11.3.1. Market Revenue and Forecast, By Type (2016-2028)
11.3.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.3.3. Market Revenue and Forecast, by End User (2016-2028)
11.3.4. Market Revenue and Forecast, By Component (2016-2028)
11.3.5. India
11.3.5.1. Market Revenue and Forecast, By Type (2016-2028)
11.3.5.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.3.5.3. Market Revenue and Forecast, by End User (2016-2028)
11.3.5.4. Market Revenue and Forecast, By Component (2016-2028)
11.3.6. China
11.3.6.1. Market Revenue and Forecast, By Type (2016-2028)
11.3.6.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.3.6.3. Market Revenue and Forecast, by End User (2016-2028)
11.3.6.4. Market Revenue and Forecast, By Component (2016-2028)
11.3.7. Japan
11.3.7.1. Market Revenue and Forecast, By Type (2016-2028)
11.3.7.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.3.7.3. Market Revenue and Forecast, by End User (2016-2028)
11.3.7.4. Market Revenue and Forecast, By Component (2016-2028)
11.3.8. Rest of APAC
11.3.8.1. Market Revenue and Forecast, By Type (2016-2028)
11.3.8.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.3.8.3. Market Revenue and Forecast, by End User (2016-2028)
11.3.8.4. Market Revenue and Forecast, By Component (2016-2028)
11.4. MEA
11.4.1. Market Revenue and Forecast, By Type (2016-2028)
11.4.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.4.3. Market Revenue and Forecast, by End User (2016-2028)
11.4.4. Market Revenue and Forecast, By Component (2016-2028)
11.4.5. GCC
11.4.5.1. Market Revenue and Forecast, By Type (2016-2028)
11.4.5.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.4.5.3. Market Revenue and Forecast, by End User (2016-2028)
11.4.5.4. Market Revenue and Forecast, By Component (2016-2028)
11.4.6. North Africa
11.4.6.1. Market Revenue and Forecast, By Type (2016-2028)
11.4.6.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.4.6.3. Market Revenue and Forecast, by End User (2016-2028)
11.4.6.4. Market Revenue and Forecast, By Component (2016-2028)
11.4.7. South Africa
11.4.7.1. Market Revenue and Forecast, By Type (2016-2028)
11.4.7.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.4.7.3. Market Revenue and Forecast, by End User (2016-2028)
11.4.7.4. Market Revenue and Forecast, By Component (2016-2028)
11.4.8. Rest of MEA
11.4.8.1. Market Revenue and Forecast, By Type (2016-2028)
11.4.8.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.4.8.3. Market Revenue and Forecast, by End User (2016-2028)
11.4.8.4. Market Revenue and Forecast, By Component (2016-2028)
11.5. Latin America
11.5.1. Market Revenue and Forecast, By Type (2016-2028)
11.5.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.5.3. Market Revenue and Forecast, by End User (2016-2028)
11.5.4. Market Revenue and Forecast, By Component (2016-2028)
11.5.5. Brazil
11.5.5.1. Market Revenue and Forecast, By Type (2016-2028)
11.5.5.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.5.5.3. Market Revenue and Forecast, by End User (2016-2028)
11.5.5.4. Market Revenue and Forecast, By Component (2016-2028)
11.5.6. Rest of LATAM
11.5.6.1. Market Revenue and Forecast, By Type (2016-2028)
11.5.6.2. Market Revenue and Forecast, By Delivery Mode (2016-2028)
11.5.6.3. Market Revenue and Forecast, by End User (2016-2028)
11.5.6.4. Market Revenue and Forecast, By Component (2016-2028)
Chapter 12. Company Profiles
12.1. Forte Research Systems Inc.
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Bioclinica
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Oracle Corporation
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Medidata Solutions Inc.
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. DATATRAK
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Medpace Holdings, Inc.
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Clinical Data Inc.
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. BioClinica
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. G.Tech Medical Engineering GmbH
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. bio-Optronics, Inc.
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
12.11. PARAXEL International Corporation.
12.11.1. Company Overview
12.11.2. Product Offerings
12.11.3. Financial Performance
12.11.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others