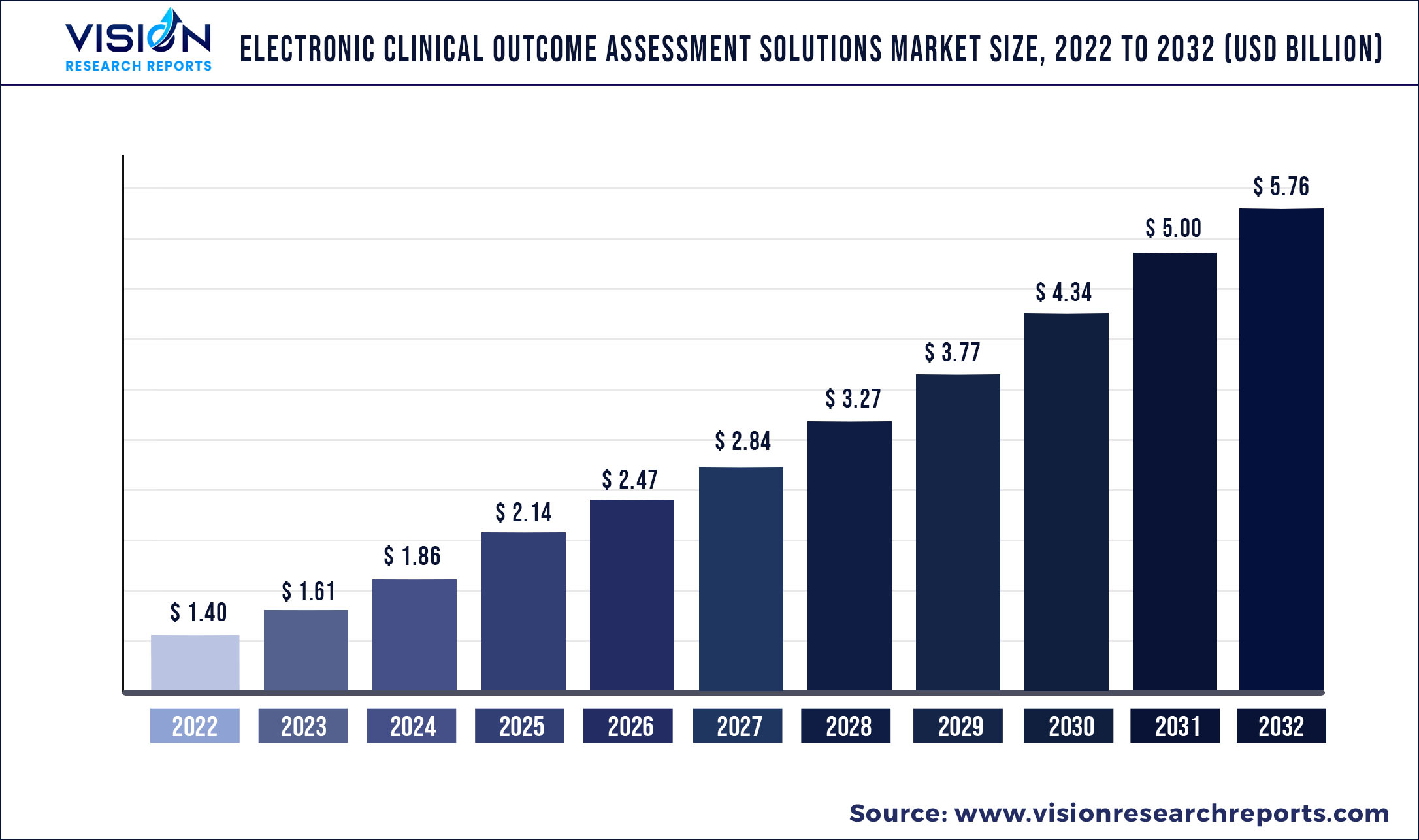
The global electronic clinical outcome assessment solutions market was valued at USD 1.40 billion in 2022 and it is predicted to surpass around USD 5.76 billion by 2032 with a CAGR of 15.2% from 2023 to 2032.
Key Pointers
| Report Coverage | Details |
| Market Size in 2022 | USD 1.40 billion |
| Revenue Forecast by 2032 | USD 5.76 billion |
| Growth rate from 2023 to 2032 | CAGR of 15.2% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Covered | IBM; IQVIA Inc.; Medidata Solutions, Inc.; Clario; ArisGlobal; Signant Health; TransPerfect; Cloudbyz; Clime do Health GmbH; ClinCapture |
An increasing number of clinical trials, the need to improve compliance, effectively capture and manage clinical information, the need to reduce costs, and increasing R&D activities, are the factors expected to drive the growth of the market. In December 2020, Kayentis- a French company specializing in electronic clinical outcome assessment (eCOA) solutions received funding of about USD 8.3 million. This facilitated its objectives for regional expansion and product development.
COVID-19 adversely impacted the conduction and management of clinical trials in 2020. However, market stakeholders implemented several measures such as the adoption of eClinical solutions to overcome these challenges and to ensure the continuity of clinical research activities. The pandemic thus helped catalyze the digitalization of clinical trials.
Many sponsors, Biopharma companies, medical device firms, & CROs began adopting eClinical solutions to collect data, manage it, and draw useful insights effectively and remotely. Electronic clinical outcomes assessments (eCOAs) support the collection of patient data remotely. This helped investigators and sponsors track patient progress during the pandemic. The trend is anticipated to continue post-COVID.
The rising adoption of eCOA by medical research professionals is driving market growth. With the rising number of research studies, the need for a centralized data capture system that helps improve patient engagement is also increasing. This is anticipated to fuel market demand during r the forecast period. The use of electronic patient-reported outcomes (ePROs) has emerged as a helpful tool during COVID-19 to collect and share important trial data, and to use other embedded tools such as alerts and reminders to weave the trial into the daily lives of the patients. This boosts patient engagement. Technological advancements such as Alexa-style tools, reward features, and gamification are estimated to enhance eCOA products thus driving market growth in the future.
Electronic-based services for data capturing & analyzing meet all the challenges of paper-based records, with an increase in patient compliance. They also eliminate the risk of data variance and minimize the site monitoring cost. Streamlined information offered by these solutions helps improvise the data quality by collecting the information in a structured way. These aforementioned benefits with the use of eCOA are expected to drive demand for the products in the coming years.
Market Segmentation
| By Delivery Mode | By End-user |
|
On-premise Web & Cloud-based |
Hospitals/Healthcare Providers CROs Pharmaceutical & Biotechnology Firms Medical Device Companies Others |
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Electronic Clinical Outcome Assessment Solutions Market
5.1. COVID-19 Landscape: Electronic Clinical Outcome Assessment Solutions Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Electronic Clinical Outcome Assessment Solutions Market, By Delivery Mode
8.1. Electronic Clinical Outcome Assessment Solutions Market, by Delivery Mode, 2023-2032
8.1.1. On-premise
8.1.1.1. Market Revenue and Forecast (2019-2032)
8.1.2. Web & Cloud-based
8.1.2.1. Market Revenue and Forecast (2019-2032)
Chapter 9. Global Electronic Clinical Outcome Assessment Solutions Market, By End-user
9.1. Electronic Clinical Outcome Assessment Solutions Market, by End-user, 2023-2032
9.1.1. Hospitals/Healthcare Providers
9.1.1.1. Market Revenue and Forecast (2019-2032)
9.1.2. CROs
9.1.2.1. Market Revenue and Forecast (2019-2032)
9.1.3. Pharmaceutical & Biotechnology Firms
9.1.3.1. Market Revenue and Forecast (2019-2032)
9.1.4. Medical Device Companies
9.1.4.1. Market Revenue and Forecast (2019-2032)
9.1.5. Others
9.1.5.1. Market Revenue and Forecast (2019-2032)
Chapter 10. Global Electronic Clinical Outcome Assessment Solutions Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.1.2. Market Revenue and Forecast, by End-user (2019-2032)
10.1.3. U.S.
10.1.3.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.1.3.2. Market Revenue and Forecast, by End-user (2019-2032)
10.1.4. Rest of North America
10.1.4.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.1.4.2. Market Revenue and Forecast, by End-user (2019-2032)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.2.2. Market Revenue and Forecast, by End-user (2019-2032)
10.2.3. UK
10.2.3.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.2.3.2. Market Revenue and Forecast, by End-user (2019-2032)
10.2.4. Germany
10.2.4.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.2.4.2. Market Revenue and Forecast, by End-user (2019-2032)
10.2.5. France
10.2.5.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.2.5.2. Market Revenue and Forecast, by End-user (2019-2032)
10.2.6. Rest of Europe
10.2.6.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.2.6.2. Market Revenue and Forecast, by End-user (2019-2032)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.3.2. Market Revenue and Forecast, by End-user (2019-2032)
10.3.3. India
10.3.3.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.3.3.2. Market Revenue and Forecast, by End-user (2019-2032)
10.3.4. China
10.3.4.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.3.4.2. Market Revenue and Forecast, by End-user (2019-2032)
10.3.5. Japan
10.3.5.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.3.5.2. Market Revenue and Forecast, by End-user (2019-2032)
10.3.6. Rest of APAC
10.3.6.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.3.6.2. Market Revenue and Forecast, by End-user (2019-2032)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.4.2. Market Revenue and Forecast, by End-user (2019-2032)
10.4.3. GCC
10.4.3.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.4.3.2. Market Revenue and Forecast, by End-user (2019-2032)
10.4.4. North Africa
10.4.4.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.4.4.2. Market Revenue and Forecast, by End-user (2019-2032)
10.4.5. South Africa
10.4.5.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.4.5.2. Market Revenue and Forecast, by End-user (2019-2032)
10.4.6. Rest of MEA
10.4.6.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.4.6.2. Market Revenue and Forecast, by End-user (2019-2032)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.5.2. Market Revenue and Forecast, by End-user (2019-2032)
10.5.3. Brazil
10.5.3.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.5.3.2. Market Revenue and Forecast, by End-user (2019-2032)
10.5.4. Rest of LATAM
10.5.4.1. Market Revenue and Forecast, by Delivery Mode (2019-2032)
10.5.4.2. Market Revenue and Forecast, by End-user (2019-2032)
Chapter 11. Company Profiles
11.1. IBM
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. IQVIA Inc.
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Medidata Solutions, Inc.
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. Clario
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. LTE Scientific
11.5. ArisGlobal
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Signant Health
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. TransPerfect
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Cloudbyz
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Clime do Health GmbH
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. ClinCapture
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others