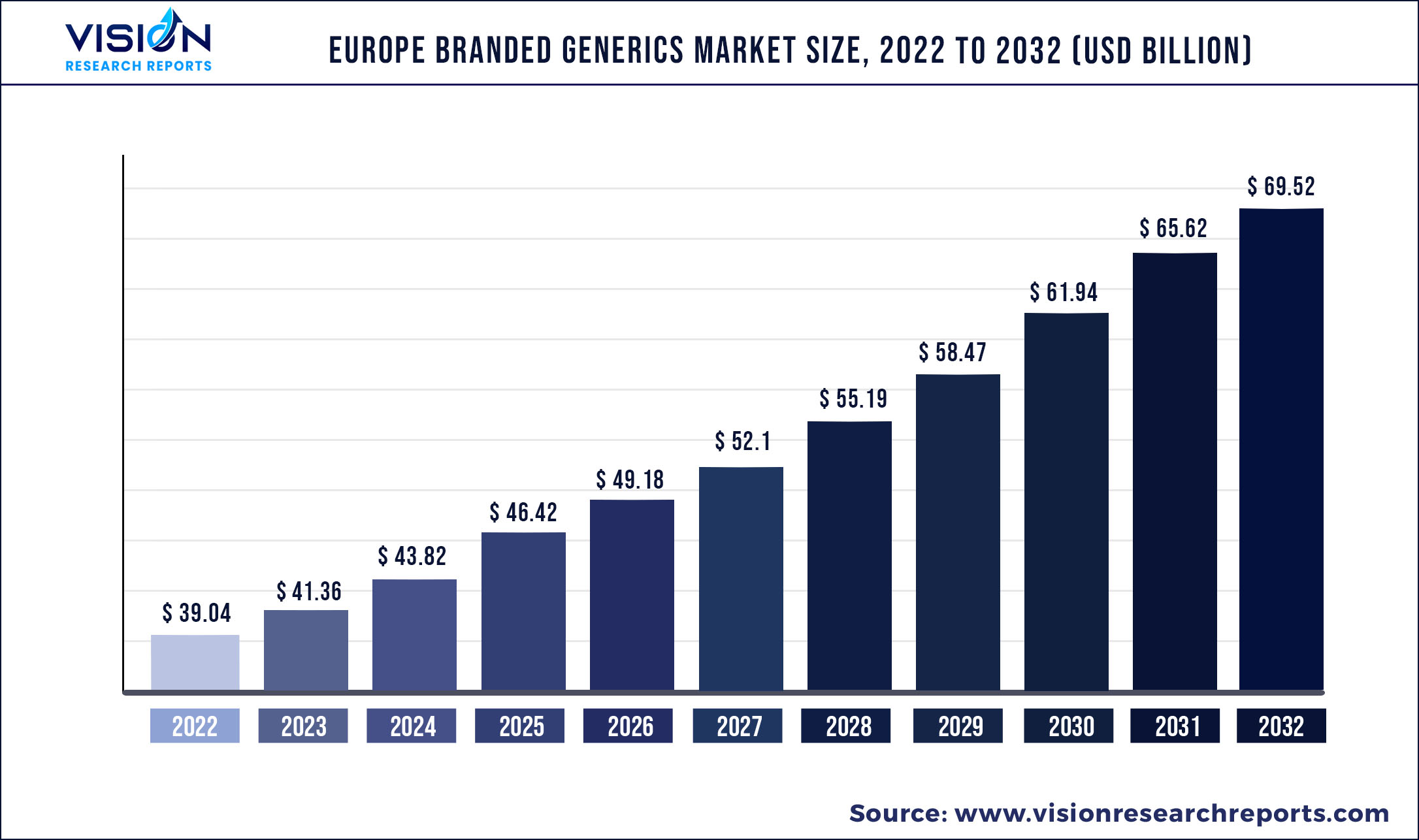
The Europe branded generics market was surpassed at USD 39.04 billion in 2022 and is expected to hit around USD 69.52 billion by 2032, growing at a CAGR of 5.94% from 2023 to 2032.
Key Players
| Report Coverage | Details |
| Market Size in 2022 | USD 39.04 billion |
| Revenue Forecast by 2032 | USD 69.52 billion |
| Growth rate from 2023 to 2032 | CAGR of 5.94% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Segmentation | Drug Class, Application, Route of Administration, Distribution Channel |
| Companies Covered | Teva Pharmaceutical Industries Ltd, Lupin, Sanofi, Sun Pharmaceutical Industries Ltd., Dr. Reddy’s Laboratories Ltd, Endo International plc., GlaxoSmithKline plc, Wockhardt, Viatris, Inc., Apotex, Inc. |
The rising prevalence of chronic diseases across the region is expected to contribute to market growth. The increasing burden of infectious & non-infectious diseases coupled with the growing geriatric population, which is more susceptible to chronic diseases, such as diabetes, hypertension, and obesity, is expected to positively impact market growth. In 2021, there were around 2.2 million people affected with HIV in WHO Europe Region, and around 57.0% of the newly diagnosed patients were from the Russian Federation. Competitive rivalry in the Europe branded generics market is likely to be high due to the various strategies adopted by the key players such as merger & acquisition and expansion of the business to strengthen their position in the market. Many established companies are engaged in the development of a generic version of branded drugs.
In October 2022, Aspire Pharma, a portfolio company of H.I.G. Capital, LLC acquired Morningside Pharmaceuticals and Morningside Healthcare, a leading provider of branded and generic specialty pharmaceuticals. Morningside has more than 80 product families of multiple therapeutic areas such as central nervous system (CNS), gastrointestinal diseases (GID), psychiatry, infectious, and endocrine. This acquisition is expected to drive Europe branded generics market.
Furthermore, in 2020, the EU strategically took steps to increase access to biosimilars and generic medicines. This included initiatives such as removing barriers that delay market entry of generics, increasing access to health systems, and digitalization of medicine regulatory systems to expedite processes ensuring ease of market entry. This initiative also boosted the production of branded generics by encouraging pharmaceutical companies to develop and manufacture these medicines and support the growth of Europe branded generics market.
The generic drug manufacturers have warned they may halt production of making low priced generic drugs due to surging electricity costs and the raising prices of drugs. For instance, in September 2022, Medicines for Europe sent an open letter to the Europe Union member states energy and health ministers regarding tackling Europe’s energy crisis, with a gas price cap on the table and a tax on profits of fossil fuel companies. This factor restrains the growth of the Europe branded generics market.
Europe Branded Generics Market Segmentations:
| By Drug Class | By Application | By Route of Administration | By Distribution Channel |
|
Alkylating Agents Antimetabolites Hormones Anti-hypertensive & Lipid lowering drugs Anti-depressants Anti-psychotics Anti-epileptics Others |
Oncology Cardiovascular Diseases Neurological Diseases Gastrointestinal Diseases Dermatological diseases Acute and Chronic Pain Others |
Topical Oral Parenteral Others |
Hospital Pharmacy Retail Pharmacy Online Pharmacy & Others |
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Europe Branded Generics Market
5.1. COVID-19 Landscape: Europe Branded Generics Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Europe Branded Generics Market, By Drug Class
8.1. Europe Branded Generics Market, by Drug Class, 2023-2032
8.1.1. Alkylating Agents
8.1.1.1. Market Revenue and Forecast (2019-2032)
8.1.2. Antimetabolites
8.1.2.1. Market Revenue and Forecast (2019-2032)
8.1.3. Hormone
8.1.3.1. Market Revenue and Forecast (2019-2032)
8.1.4. Anti-hypertensive & Lipid lowering drugs
8.1.4.1. Market Revenue and Forecast (2019-2032)
8.1.5. Anti-depressants
8.1.5.1. Market Revenue and Forecast (2019-2032)
8.1.6. Anti-psychotics
8.1.6.1. Market Revenue and Forecast (2019-2032)
8.1.7. Anti-epileptics
8.1.7.1. Market Revenue and Forecast (2019-2032)
8.1.8. Others
8.1.8.1. Market Revenue and Forecast (2019-2032)
Chapter 9. Europe Branded Generics Market, By Application
9.1. Europe Branded Generics Market, by Application, 2023-2032
9.1.1. Oncology
9.1.1.1. Market Revenue and Forecast (2019-2032)
9.1.2. Cardiovascular Diseases
9.1.2.1. Market Revenue and Forecast (2019-2032)
9.1.3. Neurological Diseases
9.1.3.1. Market Revenue and Forecast (2019-2032)
9.1.4. Gastrointestinal Diseases
9.1.4.1. Market Revenue and Forecast (2019-2032)
9.1.5. Dermatological diseases
9.1.5.1. Market Revenue and Forecast (2019-2032)
9.1.6. Acute and Chronic Pain
9.1.6.1. Market Revenue and Forecast (2019-2032)
9.1.7. Others
9.1.7.1. Market Revenue and Forecast (2019-2032)
Chapter 10. Europe Branded Generics Market, By Route of Administration
10.1. Europe Branded Generics Market, by Route of Administration, 2023-2032
10.1.1. Topical
10.1.1.1. Market Revenue and Forecast (2019-2032)
10.1.2. Oral
10.1.2.1. Market Revenue and Forecast (2019-2032)
10.1.3. Parenteral
10.1.3.1. Market Revenue and Forecast (2019-2032)
10.1.4. Others
10.1.4.1. Market Revenue and Forecast (2019-2032)
Chapter 11. Europe Branded Generics Market, By Distribution Channel
11.1. Europe Branded Generics Market, by Distribution Channel, 2023-2032
11.1.1. Hospital Pharmacy
11.1.1.1. Market Revenue and Forecast (2019-2032)
11.1.2. Retail Pharmacy
11.1.2.1. Market Revenue and Forecast (2019-2032)
11.1.3. Online Pharmacy & Others
11.1.3.1. Market Revenue and Forecast (2019-2032)
Chapter 12. Europe Branded Generics Market, Regional Estimates and Trend Forecast
12.1. Europe
12.1.1. Market Revenue and Forecast, by Drug Class (2019-2032)
12.1.2. Market Revenue and Forecast, by Application (2019-2032)
12.1.3. Market Revenue and Forecast, by Route of Administration (2019-2032)
12.1.4. Market Revenue and Forecast, by Distribution Channel (2019-2032)
12.1.5. UK
12.1.5.1. Market Revenue and Forecast, by Drug Class (2019-2032)
12.1.5.2. Market Revenue and Forecast, by Application (2019-2032)
12.1.5.3. Market Revenue and Forecast, by Route of Administration (2019-2032)
12.1.5.4. Market Revenue and Forecast, by Distribution Channel (2019-2032)
12.1.6. Germany
12.1.6.1. Market Revenue and Forecast, by Drug Class (2019-2032)
12.1.6.2. Market Revenue and Forecast, by Application (2019-2032)
12.1.6.3. Market Revenue and Forecast, by Route of Administration (2019-2032)
12.1.6.4. Market Revenue and Forecast, by Distribution Channel (2019-2032)
12.1.7. France
12.1.7.1. Market Revenue and Forecast, by Drug Class (2019-2032)
12.1.7.2. Market Revenue and Forecast, by Application (2019-2032)
12.1.7.3. Market Revenue and Forecast, by Route of Administration (2019-2032)
12.1.7.4. Market Revenue and Forecast, by Distribution Channel (2019-2032)
12.1.8. Rest of Europe
12.1.8.1. Market Revenue and Forecast, by Drug Class (2019-2032)
12.1.8.2. Market Revenue and Forecast, by Application (2019-2032)
12.1.8.3. Market Revenue and Forecast, by Route of Administration (2019-2032)
12.1.8.4. Market Revenue and Forecast, by Distribution Channel (2019-2032)
Chapter 13. Company Profiles
13.1. Teva Pharmaceutical Industries Ltd
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. Lupin
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. Sanofi
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. Sun Pharmaceutical Industries Ltd.
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. Dr. Reddy’s Laboratories Ltd
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. Endo International plc.
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. GlaxoSmithKline plc
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. Wockhardt
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. Viatris, Inc.
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. Apotex, Inc.
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others