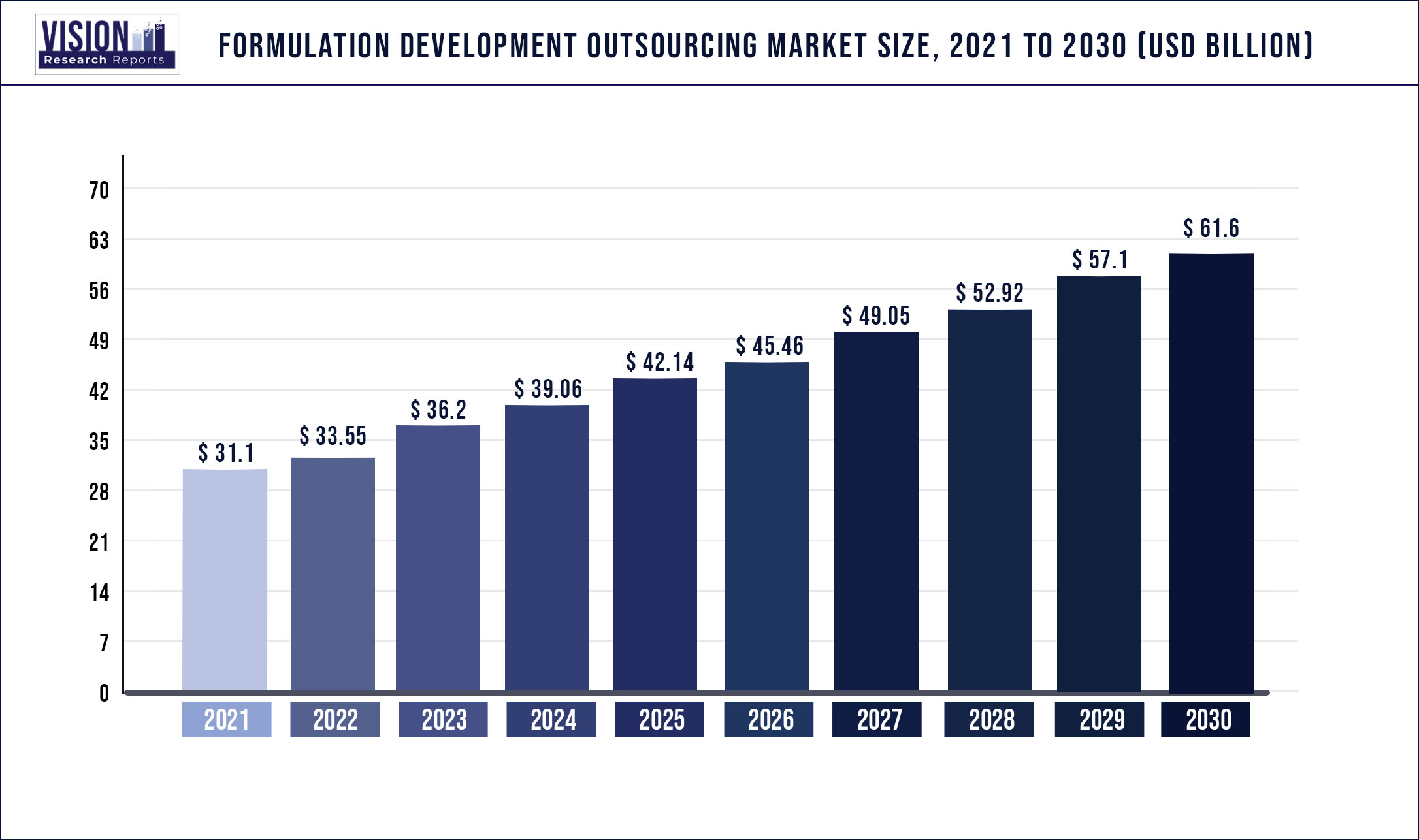
The global formulation development outsourcing market size was estimated at around USD 31.1 billion in 2021 and it is projected to hit around USD 61.6 billion by 2030, growing at a CAGR of 7.89% from 2022 to 2030.
The high burden of chronic and infectious diseases, the growing focus on improving the bioavailability of poorly soluble drugs, and the complications associated with drug development are contributing to the demand for formulation development services globally. Furthermore, the COVID-19 outbreak has influenced the need for clinical trials to find an effective treatment against the contagious virus. This has resulted in significant investments in research and development (R&D) to develop therapeutics, which are expected to drive the market.
According to Pharma R&D Annual Review 2022, biopharmaceutical and anticancer drugs are the major drugs in the development stage in the year 2022, this is expected to improve the demand for formulation development of these drugs post-pandemic. Pharmaceutical companies globally are making significant contributions to R&D activities. For instance, Merck’s R&D cost was USD 2,516.8 million in 2020 as compared to USD 2,494.8 million in 2019. Similarly, Biogen’s R&D expenses accounted for USD 3,990.9 million in 2020. The company’s R&D expenditure increased by 75% as compared to 2019. The significant increase in R&D expenditure is likely to have a positive impact on the market.
A significant number of drugs fail to reach late-stage clinical trials owing to complications associated with formulation development. Moreover, strict regulations regarding the development of drugs are further contributing to the demand for outsourcing formulation development. Such factors are expected to improve the demand for formulation development outsourcing. The COVID-19 incidence has been significantly reduced owing to the growing vaccination drive globally. As a result, the CRO and CDMO are once again concentrating on the development of drugs for oncology and other disorders. For instance, in April 2022, Labcorp collaborated with Xcell Biosciences to support the company in developing cell and gene therapies for treating cancer, Parkinson’s disease, and other rare diseases. Such initiatives by the CDMOs are likely to promote the demand for the formulation development outsourcing of drugs used in the treatment of cancer and other rare diseases.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 31.1 billion |
| Revenue Forecast by 2030 | USD 61.6 billion |
| Growth rate from 2022 to 2030 | CAGR of 7.89% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Service, formulation, therapeutic area, region |
| Companies Covered |
SGS S.A.; Intertek Group plc; Recipharm; Lonza; Charles River Laboratories International, Inc.; Eurofins Scientific SE; Element; Labcorp; Thermo Fisher Scientific, Inc. (Patheon); Catalent Inc. |
Service Insights
The formulation development segment dominated the market for formulation development outsourcing and accounted for the largest revenue share of 76.6% in 2021. Formulation development is required in each stage of clinical trials, and over the years, the number of clinical studies has increased significantly. According to ClinicalTrials.gov, there were over 293,251 research studies registered in 2019, while the number of registered studies climbed to 362,503 in 2021. The growing number of clinical studies is likely to have a positive impact on the market for formulation development outsourcing.
In February 2021, Outsourced Pharma published a survey regarding the percentage of activities outsourced by sterile injectable product manufacturers. The survey stated that over 49% of respondents outsourced formulation support services, 44% outsourced formulation optimization activities, and 52.0% outsourced process development activities. Furthermore, the survey also reported that only 45% of respondents opted for the outsourcing of pre-formulation support activities. A significant number of manufacturers outsourcing formulation development activities are likely to have a positive impact on the market for formulation development outsourcing. The high prevalence of chronic diseases such as cancer, diabetes, HIV, and other diseases is further contributing to the demand for developing innovative therapies, which is further promoting the demand for formulation development.
Formulation Insights
The oral segment dominated the formulation development outsourcing market and accounted for the maximum revenue share of 63.6% in 2021. Oral formulations include tablets, capsules, syrups, and powders, among others. Oral formulations have gained a significant share in the market as these formulations are majorly used in treating some common diseases such as migraines, fever, infectious diseases, and diabetes, among others. Oral formulations are self-administering and do not require a trained physician for drug administration, which is one of the major reasons for the high acceptance of oral formulations. Moreover, these formulations also have more flexibility in formulation design as compared to others, which further improves their demand in the market for formulation development outsourcing.
The injectable formulation segment is expected to rise with the fastest CAGR of 7.8% in the market for formulation development outsourcing over the forecast period. The high bioavailability of injectable formulations leading to the immediate onset of action is the prime factor for its fastest growth. Apart from this, injectable formulations are preferred if the drugs are poorly absorbed. Moreover, this form of formulation is also primarily preferred in cases of medical emergencies, which is further driving the segment.
Therapeutic Area Insights
Based on the therapeutic area, the oncology segment dominated the market for formulation development outsourcing and gained the largest revenue share of 24.5% in 2021. This segment is also expected to witness the fastest CAGR over the forecast period. Cancer is one of the major causes of death worldwide, which has significantly led to the demand for new cancer therapies. According to ClinicalTrials.gov, as of June 6, 2022, over 29,662 studies were in the active stage for cancer. The high number of clinical studies for cancer is likely to improve the demand for outsourcing formulation development services for cancer treatment.
Furthermore, a significant number of organizations are providing research funding for cancer. For instance, the World Cancer Research Fund International provides over GBP 300,000 for over three years for investigator-initiated trials. The NCI also provides a huge amount of funding for cancer research. For instance, in 2020, the estimated research funding for cancer accounted for USD 5370.1 million. Such initiatives by organizations are likely to have a positive impact on the market for formulation development outsourcing.
Regional Insights
Asia Pacific dominated the market for formulation development outsourcing and held the largest revenue share of 39.1% in 2021. The region is also expected to witness the fastest CAGR of 8.1% over the forecast. The presence of a significant number of CROs offering cost-effective formulation developing services is one of the major reasons for the largest market share. Furthermore, public organizations are taking initiatives to reduce the time of drug approval in the region. For instance, in October 2021, the Department of Pharmaceuticals (India) drafted new rules to reduce the time required for the approval of innovative products by at least 50% within the next two years, to improve the R&D activities in the country. Such initiatives are likely to improve the demand for formulation development outsourcing services in the country.
In North America, the market for formulation development outsourcing held a significant share of 26.8% in 2021. For instance, a significant number of clinical trials are conducted in the U.S. For instance, as of June 2022, over 33% of global clinical trials were recruited in the U.S. alone. Apart from this, public organizations in the U.S. also support the research through funding. For instance, the estimated research funding for NCI accounted for USD 6,440.4 million in 2020. The abovementioned factors are likely to promote the demand for formulation development outsourcing services in the region.
Key Players
Market Segmentation
Chapter 1. Introduction
1.1.Research Objective
1.2.Scope of the Study
1.3.Definition
Chapter 2. Research Methodology
2.1.Research Approach
2.2.Data Sources
2.3.Assumptions & Limitations
Chapter 3. Executive Summary
3.1.Market Snapshot
Chapter 4. Market Variables and Scope
4.1.Introduction
4.2.Market Classification and Scope
4.3.Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Therapeutic Area Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Formulation Development Outsourcing Market
5.1.COVID-19 Landscape: Formulation Development Outsourcing Industry Impact
5.2.COVID 19 - Impact Assessment for the Industry
5.3.COVID 19 Impact: Global Major Government Policy
5.4.Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1.Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2.Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Formulation Development Outsourcing Market, By Service
8.1.Formulation Development Outsourcing Market, by Service Type, 2022-2030
8.1.1. Preformulation
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Formulation Development
8.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Formulation Development Outsourcing Market, By Formulation
9.1.Formulation Development Outsourcing Market, by Formulation, 2022-2030
9.1.1. Oral
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Injectable
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Topical
9.1.3.1. Market Revenue and Forecast (2017-2030)
9.1.4. Others
9.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 10.Global Formulation Development Outsourcing Market, By Therapeutic Area Channel
10.1.Formulation Development Outsourcing Market, by Therapeutic Area Channel, 2022-2030
10.1.1.Oncology
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2.Infectious Diseases
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3.Neurology
10.1.3.1. Market Revenue and Forecast (2017-2030)
10.1.4.Hematology
10.1.4.1. Market Revenue and Forecast (2017-2030)
10.1.5.Respiratory
10.1.5.1. Market Revenue and Forecast (2017-2030)
10.1.6.Cardiovascular
10.1.6.1. Market Revenue and Forecast (2017-2030)
10.1.7.Dermatology
10.1.7.1. Market Revenue and Forecast (2017-2030)
10.1.8.Others
10.1.8.1. Market Revenue and Forecast (2017-2030)
Chapter 11.Global Formulation Development Outsourcing Market, Regional Estimates and Trend Forecast
11.1.North America
11.1.1.Market Revenue and Forecast, by Service (2017-2030)
11.1.2.Market Revenue and Forecast, by Formulation (2017-2030)
11.1.3.Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.1.4.U.S.
11.1.4.1. Market Revenue and Forecast, by Service (2017-2030)
11.1.4.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.1.4.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.1.5.Rest of North America
11.1.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.1.5.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.1.5.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.2.Europe
11.2.1.Market Revenue and Forecast, by Service (2017-2030)
11.2.2.Market Revenue and Forecast, by Formulation (2017-2030)
11.2.3.Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.2.4.UK
11.2.4.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.4.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.2.4.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.2.5.Germany
11.2.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.5.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.2.5.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.2.6.France
11.2.6.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.6.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.2.6.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.2.7.Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.7.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.2.7.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.3.APAC
11.3.1.Market Revenue and Forecast, by Service (2017-2030)
11.3.2.Market Revenue and Forecast, by Formulation (2017-2030)
11.3.3.Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.3.4.India
11.3.4.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.4.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.3.4.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.3.5.China
11.3.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.5.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.3.5.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.3.6.Japan
11.3.6.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.6.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.3.6.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.3.7.Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.7.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.3.7.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.4.MEA
11.4.1.Market Revenue and Forecast, by Service (2017-2030)
11.4.2.Market Revenue and Forecast, by Formulation (2017-2030)
11.4.3.Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.4.4.GCC
11.4.4.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.4.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.4.4.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.4.5.North Africa
11.4.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.5.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.4.5.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.4.6.South Africa
11.4.6.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.6.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.4.6.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.4.7.Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.7.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.4.7.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.5.Latin America
11.5.1.Market Revenue and Forecast, by Service (2017-2030)
11.5.2.Market Revenue and Forecast, by Formulation (2017-2030)
11.5.3.Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.5.4.Brazil
11.5.4.1. Market Revenue and Forecast, by Service (2017-2030)
11.5.4.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.5.4.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
11.5.5.Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.5.5.2. Market Revenue and Forecast, by Formulation (2017-2030)
11.5.5.3. Market Revenue and Forecast, by Therapeutic Area Channel (2017-2030)
Chapter 12.Company Profiles
12.1.SGS S.A.
12.1.1.Company Overview
12.1.2.Product offerings
12.1.3.Financial Performance
12.1.4.Recent Initiatives
12.2.Intertek Group plc
12.2.1.Company Overview
12.2.2.Product offerings
12.2.3.Financial Performance
12.2.4.Recent Initiatives
12.3.Recipharm
12.3.1.Company Overview
12.3.2.Product offerings
12.3.3.Financial Performance
12.3.4.Recent Initiatives
12.4.Lonza
12.4.1.Company Overview
12.4.2.Product offerings
12.4.3.Financial Performance
12.4.4.Recent Initiatives
12.5.Charles River Laboratories International, Inc.
12.5.1.Company Overview
12.5.2.Product offerings
12.5.3.Financial Performance
12.5.4.Recent Initiatives
12.6.Eurofins Scientific SE
12.6.1.Company Overview
12.6.2.Product offerings
12.6.3.Financial Performance
12.6.4.Recent Initiatives
12.7.Element
12.7.1.Company Overview
12.7.2.Product offerings
12.7.3.Financial Performance
12.7.4.Recent Initiatives
12.8.Labcorp
12.8.1.Company Overview
12.8.2.Product offerings
12.8.3.Financial Performance
12.8.4.Recent Initiatives
12.9.Thermo Fisher Scientific, Inc. (Patheon)
12.9.1.Company Overview
12.9.2.Product offerings
12.9.3.Financial Performance
12.9.4.Recent Initiatives
12.10.Catalent Inc.
12.10.1. Company Overview
12.10.2. Product offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13.Research Methodology
13.1.Primary Research
13.2.Secondary Research
13.3.Assumptions
Chapter 14.Appendix
14.1.About Us
14.2.Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others