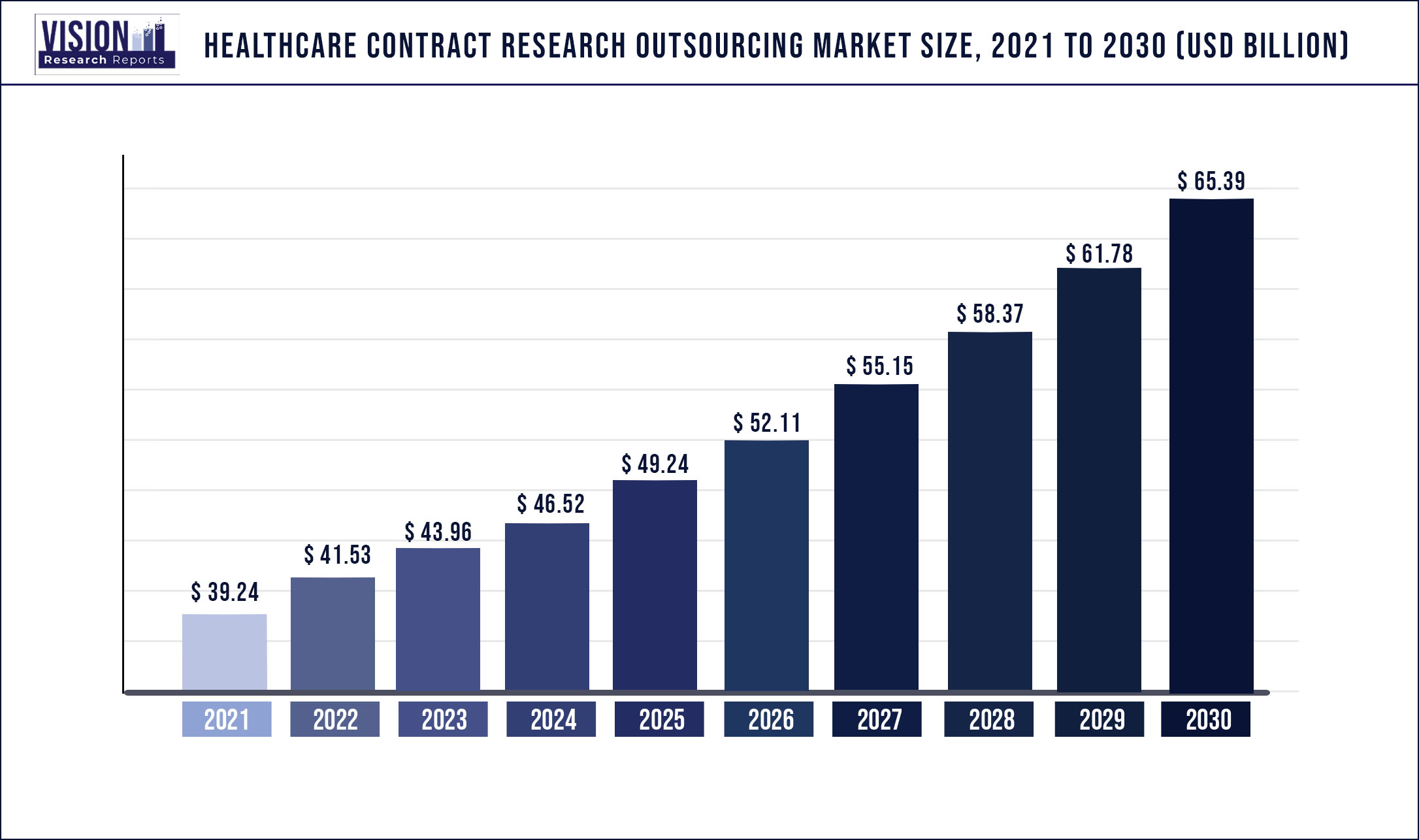
The global healthcare contract research outsourcing market was surpassed at USD 39.24 billion in 2021 and is expected to hit around USD 65.39 billion by 2030, growing at a CAGR of 5.84% from 2022 to 2030.
Healthcare Contract Research Outsourcing Market: Overview
The healthcare contract research outsourcing market is projected to witness strong growth during the forecast period driven by increase in funding for small to mid-sized pharmaceutical, biotechnology, and medical device companies, thus driving the companies to opt for CRO services with focus on niche markets. The global healthcare contract research outsourcing market is witnessing high growth, owing to globalization of clinical trials, increasing efforts for optimization of costs and development time for drug development, and rise in varied services and solutions offered by the CROs. Increased access to specialized technologies and therapeutics expertise is projected to fuel the adoption of clinical trial services in the North America during the forecast period.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 39.24 billion |
| Revenue Forecast by 2030 | USD 65.39 billion |
| Growth rate from 2022 to 2030 | CAGR of 5.84% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Service, Therapeutic Area, End-user, Region |
| Companies Covered | Syneos Health, PAREXEL International, ICON plc, PRA Health Sciences, Inc., Charles River, Laboratory Corporation of America Holdings (Covance), IQVIA, Medpace, Pharmaceutical Product Development, LLC. |
Incremental Opportunities for Healthcare Contract Research Outsourcing Market Players
The global healthcare contract research outsourcing market is expected to expand on the back of several factors including surge in efforts intended for costs & drug development time optimization, increase in different services and solutions provided by the CROs, and globalization of clinical trials. Some of the important services available in the healthcare contract research outsourcing market include regulatory service, clinical trial service, pharmacovigilance, site management protocol, and medical writing. These services are mainly used by biotechnology companies, pharmaceutical companies, academic institutes & government organizations, and medical device companies across the globe.
Enterprises from the global healthcare contract research outsourcing market are estimated to observe increase in demand for regulatory services outsourcing. This increase can be attributed to rise in scrutiny by regulatory authorities, including the EMA and FDA at all stages of clinical trials. Moreover, changing regulatory needs in each region is expected to drive the demand opportunities for regulatory services outsourcing activities.
Expansion of Companies to Gain Potential Revenue Benefits Boosts Market Growth
Companies in the healthcare contract research outsourcing market are entering into collaboration and acquisition agreements in order to maintain their leading market position and expand their presence in newer regions. Some of the key players operating in the global healthcare contract research outsourcing market are focusing on offering quality services to gain attention from consumers. Outsourcing improves the global reach with respect to drug development with minimum maintenance cost, as CROs are well-versed with regulatory requirements of various regions to fueling the expansion of healthcare contract research outsourcing market. Healthcare companies often outsource drug development functions to contract research organizations to efficiently and effectively use the development capabilities internationally.
North America Dominates Global Healthcare Contract Research Outsourcing Market
North America is expected to offer lucrative opportunities due to factors such as presence of advanced infrastructure required for clinical researches and key government incentive programs in the region, specifically in the U.S. In addition, increase in availability of therapeutics expertise and specialized technologies is expected to result in rise in the number of clinical trial services in the U.S. in the upcoming years. The North America healthcare contract research outsourcing market is rising with increasing complex regulatory process & growing regulatory burden. The market in North America is primarily driven by advanced infrastructure of clinical research sites, and effective government incentive programs in United States. The Asia Pacific region is projected to be fastest growing region in the global healthcare contract research outsourcing market during the forecast period. In Asia Pacific, China and India are significant markets for healthcare CRO and India is estimated to grow at a rapid pace during the forecast period.
CROs to Contribute to Success of Pharmaceutical Industry
The clinical, therapeutic, and regulatory expertise of CROs help to improve the complete cost of development and accelerate time to market a new product. CROs have become larger and enormously more diversified across various therapeutic areas and functions, expanding their penetration. Small pharmaceutical companies are also looking for advancements in product development through CRO services due to rise in demand for innovative products and patent expiration. CROs are anticipated to expand geographically and add competencies in emerging therapeutic areas, while boosting their current services beyond traditional clinical capabilities, including post-approval, commercial, informatics, etc. where low - outsourced penetration observed, further enhancing the demand for CROs, driving greater outsourcing demand. The regulatory services outsourcing is anticipated to increase due to increased scrutiny by regulatory bodies such as the FDA and EMA at every stage of clinical trial and changing regulatory requirements as per the region.
Late Stage Drug Development Outsourcing: Key Driver
Oncology/Hematology Segment to Dominate Global Market
North America to Dominate Healthcare Contract Research Outsourcing Market
Key Players
Market Segmentation
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Healthcare Contract Research Outsourcing Market
5.1. COVID-19 Landscape: Healthcare Contract Research Outsourcing Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Healthcare Contract Research Outsourcing Market, By Service
8.1. Healthcare Contract Research Outsourcing Market, by Service, 2022-2030
8.1.1 Clinical Trial Service
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Regulatory Service
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Clinical Data Management & Biometrics
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Medical Writing
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Pharmacovigilance
8.1.5.1. Market Revenue and Forecast (2017-2030)
8.1.6. Site Management Protocol
8.1.6.1. Market Revenue and Forecast (2017-2030)
8.1.7. Others
8.1.7.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Healthcare Contract Research Outsourcing Market, By Therapeutic Area
9.1. Healthcare Contract Research Outsourcing Market, by Therapeutic Area, 2022-2030
9.1.1. Oncology / Hematology
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. CNS
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. CV / Metabolic
9.1.3.1. Market Revenue and Forecast (2017-2030)
9.1.4. Respiratory
9.1.4.1. Market Revenue and Forecast (2017-2030)
9.1.5. Infectious Diseases
9.1.5.1. Market Revenue and Forecast (2017-2030)
9.1.6. Immunology
9.1.6.1. Market Revenue and Forecast (2017-2030)
9.1.7. Rare Diseases
9.1.7.1. Market Revenue and Forecast (2017-2030)
9.1.8. Medical Devices
9.1.8.1. Market Revenue and Forecast (2017-2030)
9.1.9. Others
9.1.9.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Healthcare Contract Research Outsourcing Market, By End-user
10.1. Healthcare Contract Research Outsourcing Market, by End-user, 2022-2030
10.1.1. Pharmaceutical Companies
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Biotechnology Companies
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Medical Device Companies
10.1.3.1. Market Revenue and Forecast (2017-2030)
10.1.4. Academic Institutes & Government Organizations
10.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Healthcare Contract Research Outsourcing Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Service (2017-2030)
11.1.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.1.3. Market Revenue and Forecast, by End-user (2017-2030)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Service (2017-2030)
11.1.4.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.1.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.1.5.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.1.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.2.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.4.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.2.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.5.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.2.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.6.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.2.6.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Service (2017-2030)
11.2.7.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.2.7.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.3.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.4.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.3.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.5.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.3.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.6.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.3.6.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Service (2017-2030)
11.3.7.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.3.7.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.4.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.4.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.5.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.4.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.6.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.4.6.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Service (2017-2030)
11.4.7.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.4.7.3. Market Revenue and Forecast, by End-user (2017-2030)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.5.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Service (2017-2030)
11.5.4.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.5.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Service (2017-2030)
11.5.5.2. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
11.5.5.3. Market Revenue and Forecast, by End-user (2017-2030)
Chapter 12. Company Profiles
12.1. PAREXEL International
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. ICON plc
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. PRA Health Sciences, Inc.
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Charles River
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Laboratory Corporation of America Holdings (Covance)
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. IQVIA
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. IQVIA
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Pharmaceutical Product Development, LLC.
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others