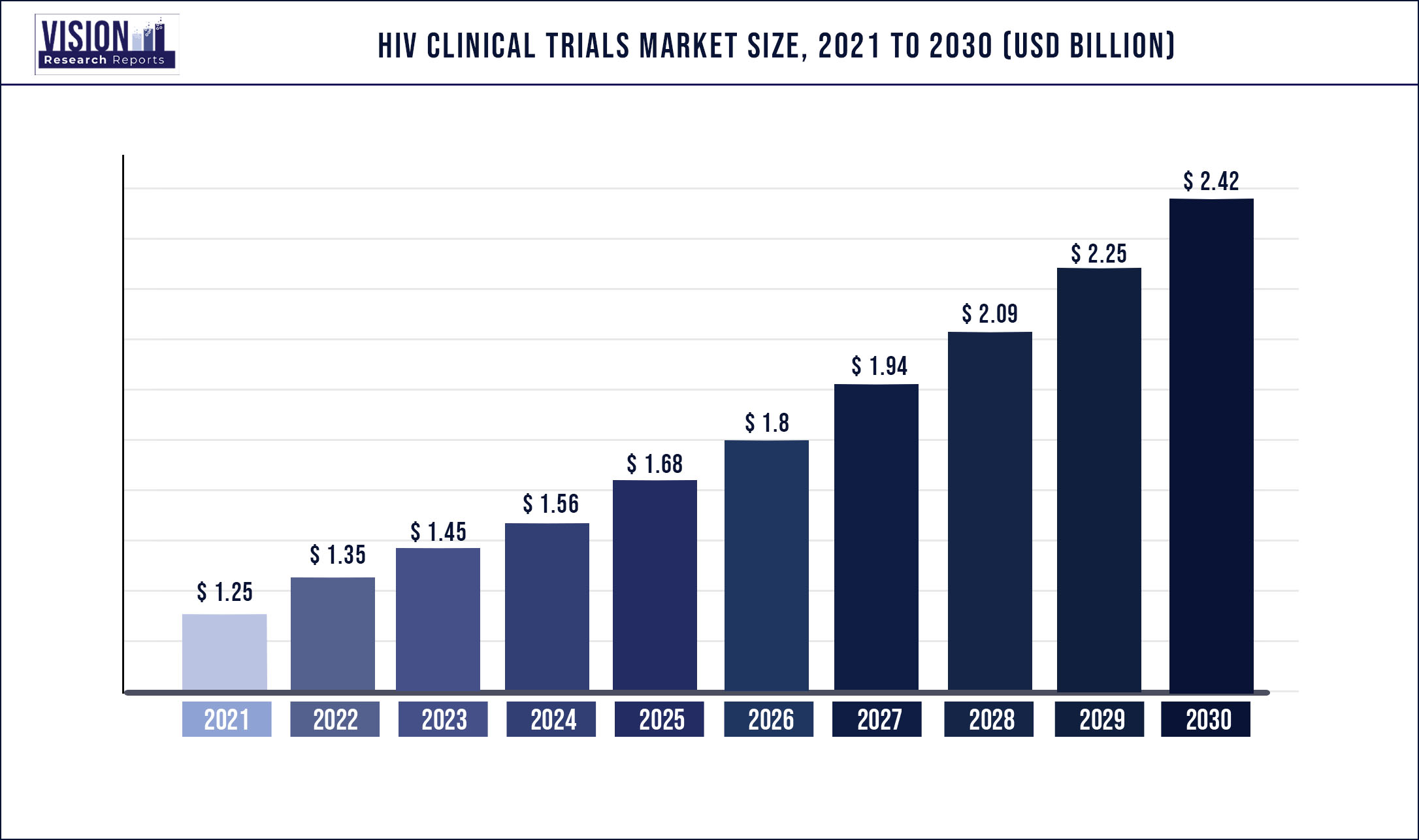
The global HIV clinical trials market was estimated at USD 1.25 billion in 2021 and it is expected to surpass around USD 2.42 billion by 2030, poised to grow at a CAGR of 7.62% from 2022 to 2030
Report Highlights
The main drivers of this market are increasing HIV vaccine trials, increased R&D activities for HIV clinical trials, and rising HIV infection awareness, which leads to a large number of patients enrolling in clinical trials. During the COVID-19 pandemic, there was a halt in the market growth.
This decrease in HIV and AIDS clinical trials is due to stay-at-home orders by the government to prevent COVID-19 that led to canceled and delayed trials. It also includes disruption in patient recruitment, hurdles in the conduction of clinical trials, and rapid study for the development of vaccines and medical products for the prevention of COVID-19 that lead to detrimental effects in HIV clinical trial studies. However, these impacts were significantly reduced by adopting strategic measures taken by the clinical trial conducting organization, regulatory authorities, and government bodies.
According to the WHO, in 2020, there were around 37.7 million people infected with HIV infection. To reduce this number, increasing funding and partnerships by biotechnology and pharmaceutical firms have been observed. Moreover, increasing initiatives are being taken by the government to find treatment and spread awareness regarding this disease. For instance, in October 2021, Samsung BioLogics collaborated with Enzolytics Inc. This partnership aims to manufacture Anti-HIV and Anti-SARS-CoV-2 Monoclonal Antibody Therapies.
However, a lack of awareness regarding HIV clinical research trials and the availability of treatments for preventing it are factors that are hindering the growth of this market. According to a report by BioMed Central Ltd. in Europe, out of 467 participants, about one-third of them were not aware of the HIV infection. As a result, ignorance and unawareness regarding the disease will prevent admission to an HIV clinical trial.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 1.25 billion |
| Revenue Forecast by 2030 | USD 2.42 billion |
| Growth rate from 2022 to 2030 | CAGR of 7.62% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Phase, study design, sponsor, Region |
| Companies Covered | PPD Inc.; IQVIA Inc.; Parexel International Corporation; ICON plc; Syneos Health; WuXi AppTec; Janssen Global Services, LLC; Gilead Sciences, Inc.; Bionor Holding AS; Charles River Laboratories; GSK plc.; SGS SA |
Phase Insights
Phase I held the largest revenue share of over 30.06% in 2021. This segment is also anticipated to register the fastest CAGR during the forecast period. Phase I studies assess the safety of HIV drugs and involve the evaluation of tolerability and pharmacokinetics of molecules. It determines the effect of HIV drugs on humans including the way it is absorbed, metabolized, and excreted. It also examines the side effects of the drug in case of the increased dosage level. The stage includes 20 to 100 healthy volunteers or people with the disease.
The Phase II segment is expected to register the second-fastest CAGR of 6.62% over the forecast period. This is due to the increasing investments in the R&D of HIV clinical trials by industry and non-industry sponsors. The growing number of industry-sponsored and non-industry-sponsored clinical trials in phase II, the complexity associated with phase II clinical trials, and the globalization of clinical trials are factors expected to drive the market.
Sponsor Insights
The pharmaceutical and biopharmaceutical companies dominated the market and held a revenue share of over 70.36% in 2021. It is also expected to register the fastest CAGR during the forecast period. This can be largely attributed to the rising R&D investments and the introduction of new drugs for HIV prevention, which have increased in the past two decades. Based on sponsors, this segment is segmented into pharmaceutical and biopharmaceutical companies, non-profit organizations, and others. Others include government institutes, academics, and research centers.
The non-profit organizations' segment is expected to register the second-highest CAGR of 5.62% during the forecast period. This growth is due to the increasing staff, reinvesting the revenue generated for new drug discovery for HIV treatment, and improving service. They are also searching for new ways to conduct trials for treating HIV infection.
Study Design Insights
The interventional studies segment held the largest revenue share of over 45.17% in 2021 and it is expected to register the second-fastest CAGR during the forecast period. Intervention studies help in determining cost-effective, scalable, and preventive measures to treat HIV infection. It is also used to determine the weaknesses and strengths of a trial, estimate the impact of the treatment on individuals, and minimize limitations at the initial stage.
Based on study design, the market is segmented into interventional studies, observational studies, and expanded access studies. The expanded access studies segment is expected to expand at the fastest CAGR of 6.5% over the forecast period. The main driver is increasing innovation for HIV treatment. Expanded access is expected to be a good approach for patients with serious, life-threatening HIV conditions. In this, the patient is allowed to have treatment outside of a clinical trial when the result of the trial is not satisfactory or no improvement is shown.
Regional Insights
North America dominated the market with a revenue share of over 45.03% in 2021 and is expected to exhibit a significant CAGR over the forecast period. This market is likely to grow due to the high number of HIV clinical trials being conducted in the region. Major R&D investments and government support for HIV clinical trials are further promoting the market growth.
The Asia Pacific region is anticipated to register the fastest CAGR of 8.76% during the forecast period. Developed clinical research infrastructure, a strong hospital network, and the availability of skilled medical practitioners for HIV prevention are factors supporting the growth of the Asia Pacific market. In addition, the large and diverse patient pools that are infected with HIV in these countries lead to market growth.
Key Players
Market Segmentation
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on HIV Clinical Trials Market
5.1. COVID-19 Landscape: HIV Clinical Trials Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global HIV Clinical Trials Market, By Phase
8.1. HIV Clinical Trials Market, by Phase, 2022-2030
8.1.1 Phase I
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Phase II
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Phase III
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Phase IV
8.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global HIV Clinical Trials Market, By Study Design
9.1. HIV Clinical Trials Market, by Study Design, 2022-2030
9.1.1. Interventional Studies
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Observational Studies
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Expanded Access Studies
9.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global HIV Clinical Trials Market, By Sponsor
10.1. HIV Clinical Trials Market, by Sponsor, 2022-2030
10.1.1. Pharmaceutical & Biopharmaceutical Companies
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Non Profit Organizations
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Others
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global HIV Clinical Trials Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Phase (2017-2030)
11.1.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.1.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Phase (2017-2030)
11.1.4.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.1.4.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Phase (2017-2030)
11.1.5.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.1.5.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Phase (2017-2030)
11.2.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.2.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Phase (2017-2030)
11.2.4.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.2.4.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Phase (2017-2030)
11.2.5.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.2.5.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Phase (2017-2030)
11.2.6.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.2.6.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Phase (2017-2030)
11.2.7.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.2.7.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Phase (2017-2030)
11.3.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.3.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Phase (2017-2030)
11.3.4.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.3.4.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Phase (2017-2030)
11.3.5.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.3.5.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Phase (2017-2030)
11.3.6.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.3.6.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Phase (2017-2030)
11.3.7.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.3.7.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Phase (2017-2030)
11.4.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.4.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Phase (2017-2030)
11.4.4.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.4.4.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Phase (2017-2030)
11.4.5.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.4.5.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Phase (2017-2030)
11.4.6.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.4.6.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Phase (2017-2030)
11.4.7.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.4.7.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Phase (2017-2030)
11.5.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.5.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Phase (2017-2030)
11.5.4.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.5.4.3. Market Revenue and Forecast, by Sponsor (2017-2030)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Phase (2017-2030)
11.5.5.2. Market Revenue and Forecast, by Study Design (2017-2030)
11.5.5.3. Market Revenue and Forecast, by Sponsor (2017-2030)
Chapter 12. Company Profiles
12.1. PPD Inc.
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. IQVIA Inc.
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Parexel International Corporation
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. ICON plc
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Syneos Health
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. WuXi AppTec
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Janssen Global Services, LLC
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Gilead Sciences, Inc.
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Bionor Holding AS
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Charles River Laboratories
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others