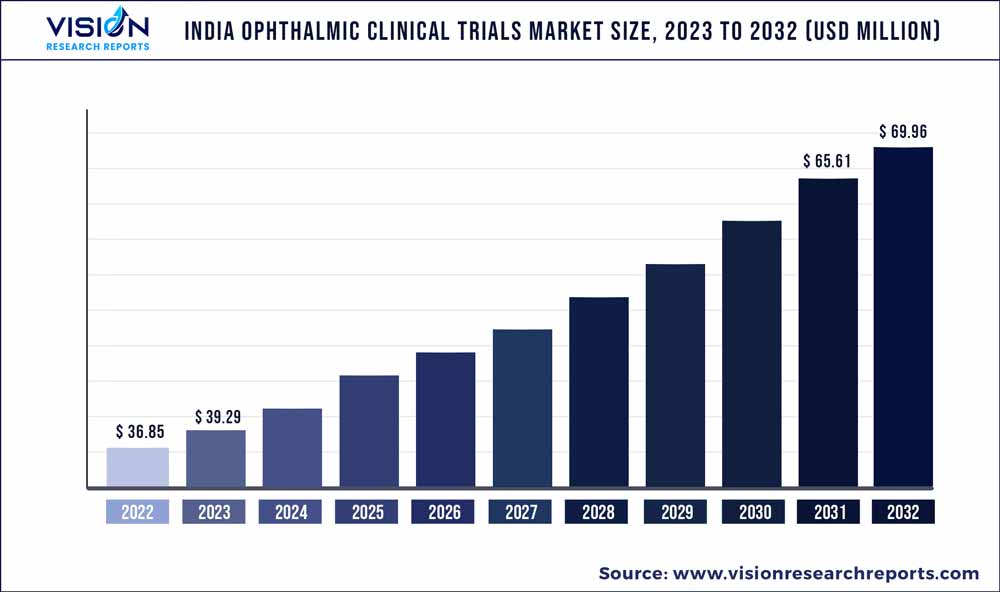
The India ophthalmic clinical trials market was estimated at USD 36.85 million in 2022 and it is expected to surpass around USD 69.96 million by 2032, poised to grow at a CAGR of 6.62% from 2023 to 2032.
Key Pointers
Report Scope of the India Ophthalmic Clinical Trials Market
| Report Coverage | Details |
| Market Size in 2022 | USD 36.85 million |
| Revenue Forecast by 2032 | USD 69.96 million |
| Growth rate from 2023 to 2032 | CAGR of 6.62% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Companies Covered | Abiogenesis Clinpharm Pvt Ltd; Veeda Clinical Research; Catawba Research, LLC.; Novotech; Catalyst Clinical Services Pvt. Ltd.; Navitas Life Sciences; Vedic Lifesciences Pvt Ltd.; Vimta Labs Ltd. |
A favorable regulatory environment, rising demand for ocular treatment therapies, and government support are key growth drivers for the market. India has made a significant effort to streamline its regulatory framework for clinical trials, aiming to attract more research investment. Moreover, a substantial educated patient population pool along with healthcare facilities is anticipated to provide further growth opportunities for this market in the coming years.
The outbreak of COVID-19 had a major impact on the way clinical trials are administered and done in India due to the push for medical needs imposed by the pandemic. To prioritize resources for COVID-19 patients during the pandemic, numerous non-urgent or elective ophthalmic treatments, such as cataract surgeries and refractive surgeries, were delayed or canceled. This led to a reduction in the number of ophthalmic procedures performed, impacting the market revenue.
On the other hand, the increasing prevalence of eye disorders such asglaucoma, dry eye disease, retinopathy, and cataract are expected to create growth opportunities for the ophthalmic clinical trials market. Glaucoma is the third most common reason for blindness in India. The affected population totals 12 million, or 12.8% of the nation's blindness. Glaucoma prevalence in India varies from 2.6% to 4.1%. Glaucoma-related blindness is permanent, and 50% of those who have it are unaware of it. Clinical studies are essential for expanding the understanding of glaucoma and creating novel techniques for its detection, treatment, and prevention.
Moreover, a favorable government environment is also anticipated to drive market growth in the forecast period. The implementation of initiatives like the establishment of ethical review committees has improved the effectiveness and transparency of the clinical trial approval procedure. Moreover, Indian governments have used a variety of strategies, such as targeted direct funding and industrial tax refunds, to try to increase the number of clinical trials in the country. This development contributed to the growth of the ophthalmic clinical trials industry in India.
Cost competitiveness is a significant factor that attracts players in the clinical trials sector to India. In India, clinical trial costs are one-tenth of the cost in the U.S. The amount spent on research and development in the pharmaceutical sector has increased dramatically due to numerous pharmaceutical corporations moving their clinical trial operations to India or outsourcing activities there. The regulatory organizations in India have established timelines to ensure the approval of trial applications in a comparatively short amount of time.
Product Insights
Based on product, the drugs/pharmaceuticals segment accounted for the largest market share of 74% in 2022. The growing prevalence of eye disorders and the increasing demand for effective treatment options have propelled pharmaceutical companies to invest significantly in ophthalmic drug development. Clinical trials are a critical step in the drug development process, enabling pharmaceutical companies to test the safety and efficacy of their ophthalmic products. India, with its large patient pool, diverse population, and well-established clinical research infrastructure, has emerged as an attractive destination for conducting ophthalmic clinical trials. Thus, these factors are driving the growth of India’s ophthalmic clinical trials industry.
The devices segment is anticipated to register lucrative growth during the forecast period. Advancements in technology have led to the development of innovative ophthalmic devices, ranging from diagnostic tools to surgical instruments. These devices play a crucial role in the diagnosis, monitoring, and treatment of various eye disorders. Additionally, the growing demand for advanced ophthalmic devices and the increasing prevalence of eye diseases are driving the need for clinical trials in India.
Phase Insights
The clinical phase segment dominated the market in 2022 and accounted for the largest revenue share of 83%. The participation of pharmaceutical companies, research organizations, and healthcare institutions in different phases of clinical trials is driving the growth of the market. The diverse patient population in India allows for a comprehensive evaluation of the investigational products, ensuring their effectiveness across different genetic and environmental factors.
Furthermore, the preclinical phase segment is expected to grow at a substantial CAGR during the forecast period. The increasing emphasis on the safety and efficacy assessment of ophthalmic drugs and devices before proceeding to human trials has amplified the demand for robust pre-clinical studies. This segment plays a crucial role in identifying potential candidates, optimizing formulations, and assessing the safety profile of investigational products, thereby reducing the risk of adverse effects during subsequent clinical phases.
Indication Insights
The retinopathy segment dominated the market for ophthalmic clinical trials with the largest revenue share of 25% in 2022. The increase in retinopathy-related research and development activities and the rising prevalence of retinopathy in the country are some of the factors driving the growth of the segment. According to a survey published by the Union Health Ministry in 2022, over 17% of Indians have diabetic retinopathy, with 3.6% of cases posing a potential threat to vision. A study has found that 10.4% of rural residents had diabetes, and about 10.3% had diabetic retinopathy. According to a WHO study, there would be a more than 50% increase in diabetics with retinal damage by the year 2032.
Furthermore, the glaucoma segment is expected to grow at a substantial CAGR during the forecast period. High prevalence of glaucoma, raising public awareness and knowledge of glaucoma are major factors propelling the market growth. The Indian government has recognized the burden of glaucoma and the requirement for ophthalmic research and development. The National Program for Control of Blindness and Visual Impairment is one initiative that aims to advance clinical research and eye care in the nation.
Service Type Insights
The clinical trial data management services segment dominated the India ophthalmic clinical trials market and accounted for the largest revenue share of 34% in 2022. Clinical trial data management services are crucial for ensuring the accuracy and integrity of data. They implement quality control measures and standardized processes to validate, collect, clean, and analyze the data generated during clinical trials.
Furthermore, bioanalytical testing services are expected to hold a significant market share in the coming years. Determining the drug release, distribution, and bioavailability in ocular tissues involves the use of bioanalytical testing services. This information aids in improving the design and development of ocular drug delivery systems for improved therapeutic results.
Sponsor Type Insights
The pharmaceutical/biopharmaceutical companies dominated the market in 2022 and accounted for the largest share of 42% of the revenue. The rising number of entities focusing on the R&D of novel ophthalmic drugs is a major factor driving the market growth. Moreover, these entities offer a broad range of products, including ophthalmic therapies and drugs. Conducting clinical trials is an essential step in the development and approval process of new drugs, formulations, and treatment modalities.
The other segment, which includes CROs and medical institutes is poised to register a lucrative CAGR during the forecast period. Medical institutes, including hospitals, universities, and research institutions, are centers of academic research in the field of ophthalmology. These institutions have specialized divisions and research facilities devoted to ocular studies. Clinical trials inside medical institutions are expanding due to advances in scientific understanding of eye illnesses and treatment options.
India Ophthalmic Clinical Trials Market Segmentations:
By Product
By Indication
By Phase
By Service Type
By Sponsor Type
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on India Ophthalmic Clinical Trials Market
5.1. COVID-19 Landscape: India Ophthalmic Clinical Trials Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. India Ophthalmic Clinical Trials Market, By Product
8.1. India Ophthalmic Clinical Trials Market, by Product, 2023-2032
8.1.1. Devices
8.1.1.1. Market Revenue and Forecast (2020-2032)
8.1.2. Drugs/Pharmaceuticals
8.1.2.1. Market Revenue and Forecast (2020-2032)
Chapter 9. India Ophthalmic Clinical Trials Market, By Indication
9.1. India Ophthalmic Clinical Trials Market, by Indication, 2023-2032
9.1.1. Macular Degeneration
9.1.1.1. Market Revenue and Forecast (2020-2032)
9.1.2. Glaucoma
9.1.2.1. Market Revenue and Forecast (2020-2032)
9.1.3. Dry Eye Disease
9.1.3.1. Market Revenue and Forecast (2020-2032)
9.1.4. Retinopathy
9.1.4.1. Market Revenue and Forecast (2020-2032)
9.1.5. Uveitis
9.1.5.1. Market Revenue and Forecast (2020-2032)
9.1.6. Macular Edema
9.1.6.1. Market Revenue and Forecast (2020-2032)
9.1.7. Blepharitis
9.1.7.1. Market Revenue and Forecast (2020-2032)
9.1.8. Cataract
9.1.8.1. Market Revenue and Forecast (2020-2032)
9.1.9. Optic Neuropathy
9.1.9.1. Market Revenue and Forecast (2020-2032)
9.1.10. Others
9.1.10.1. Market Revenue and Forecast (2020-2032)
Chapter 10. India Ophthalmic Clinical Trials Market, By Phase
10.1. India Ophthalmic Clinical Trials Market, by Phase, 2023-2032
10.1.1. Discovery Phase
10.1.1.1. Market Revenue and Forecast (2020-2032)
10.1.2. Preclinical Phase
10.1.2.1. Market Revenue and Forecast (2020-2032)
10.1.3. Clinical Phase
10.1.3.1. Market Revenue and Forecast (2020-2032)
Chapter 11. India Ophthalmic Clinical Trials Market, By Service Type
11.1. India Ophthalmic Clinical Trials Market, by Service Type, 2023-2032
11.1.1. Protocol Designing
11.1.1.1. Market Revenue and Forecast (2020-2032)
11.1.2. Site Identification
11.1.2.1. Market Revenue and Forecast (2020-2032)
11.1.3. Patient Recruitment
11.1.3.1. Market Revenue and Forecast (2020-2032)
11.1.4. Laboratory Services
11.1.4.1. Market Revenue and Forecast (2020-2032)
11.1.5. Bioanalytical Testing Services
11.1.5.1. Market Revenue and Forecast (2020-2032)
11.1.6. Clinical Trial Data Management Services
11.1.6.1. Market Revenue and Forecast (2020-2032)
11.1.7. Others
11.1.7.1. Market Revenue and Forecast (2020-2032)
Chapter 12. India Ophthalmic Clinical Trials Market, By Sponsor Type
12.1. India Ophthalmic Clinical Trials Market, by Sponsor Type, 2023-2032
12.1.1. Pharmaceutical/Biopharmaceutical Companies
12.1.1.1. Market Revenue and Forecast (2020-2032)
12.1.2. Medical Device Companies
12.1.2.1. Market Revenue and Forecast (2020-2032)
12.1.3. Others
12.1.3.1. Market Revenue and Forecast (2020-2032)
Chapter 13. India Ophthalmic Clinical Trials Market, Regional Estimates and Trend Forecast
13.1. India
13.1.1. Market Revenue and Forecast, by Product (2020-2032)
13.1.2. Market Revenue and Forecast, by Indication (2020-2032)
13.1.3. Market Revenue and Forecast, by Phase (2020-2032)
13.1.4. Market Revenue and Forecast, by Service Type (2020-2032)
13.1.5. Market Revenue and Forecast, by Sponsor Type (2020-2032)
Chapter 14. Company Profiles
14.1. Abiogenesis Clinpharm Pvt Ltd
14.1.1. Company Overview
14.1.2. Product Offerings
14.1.3. Financial Performance
14.1.4. Recent Initiatives
14.2. Veeda Clinical Research
14.2.1. Company Overview
14.2.2. Product Offerings
14.2.3. Financial Performance
14.2.4. Recent Initiatives
14.3. Catawba Research, LLC.
14.3.1. Company Overview
14.3.2. Product Offerings
14.3.3. Financial Performance
14.3.4. Recent Initiatives
14.4. Novotech
14.4.1. Company Overview
14.4.2. Product Offerings
14.4.3. Financial Performance
14.4.4. Recent Initiatives
14.5. Catalyst Clinical Services Pvt. Ltd.
14.5.1. Company Overview
14.5.2. Product Offerings
14.5.3. Financial Performance
14.5.4. Recent Initiatives
14.6. Navitas Life Sciences
14.6.1. Company Overview
14.6.2. Product Offerings
14.6.3. Financial Performance
14.6.4. Recent Initiatives
14.7. Vedic Lifesciences Pvt Ltd.
14.7.1. Company Overview
14.7.2. Product Offerings
14.7.3. Financial Performance
14.7.4. Recent Initiatives
14.8. Vimta Labs Ltd.
14.8.1. Company Overview
14.8.2. Product Offerings
14.8.3. Financial Performance
14.8.4. Recent Initiatives
Chapter 15. Research Methodology
15.1. Primary Research
15.2. Secondary Research
15.3. Assumptions
Chapter 16. Appendix
16.1. About Us
16.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others