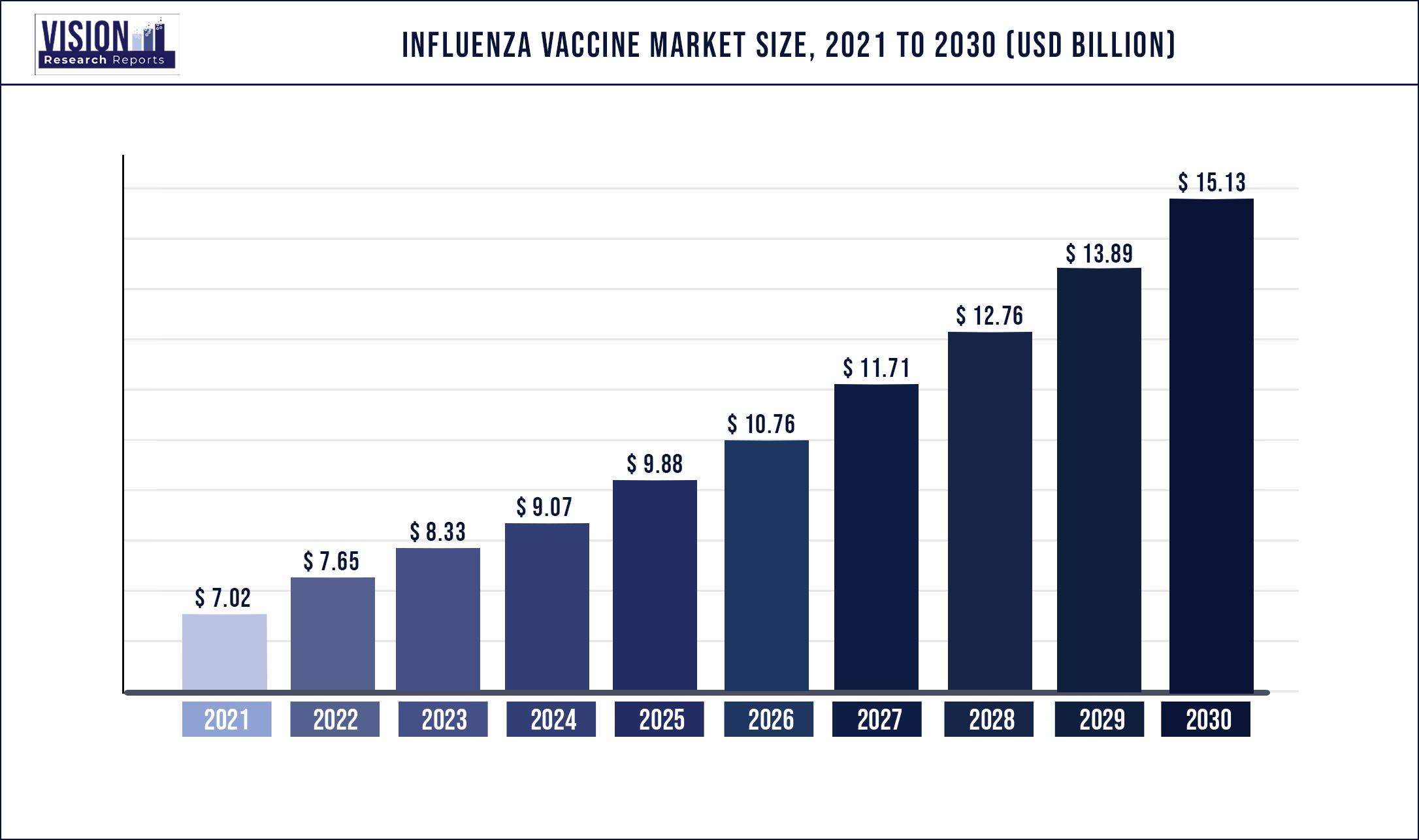
The global influenza vaccine market was estimated at USD 7.02 billion in 2021 and it is expected to surpass around USD 15.13 billion by 2030, poised to grow at a CAGR of 8.91% from 2022 to 2030.
Influenza is a major global public health concern and according to WHO, affects around 100 million individuals annually, causing around 5 million cases of severe illness and around 600,000 deaths globally along with a huge economic burden. Vaccination is crucial to slow down the incidence and to lower the severity of pandemic situations caused by viral infections.
There was an inadvertent slowdown in vaccination drives across the globe due to the incidence of COVID-19 pandemic situation. Nevertheless, many countries launched joint vaccination drives against COVID-19 and seasonal influenza together. WHO recommends such joint vaccination drives due to better reach and other potential advantages. Many pharmaceutical giants expanded their collaborations made for the development of COVID-19 vaccines to include indications for respiratory and influenza viruses. For instance, in November 2022, Arcturus Therapeutics Holdings Inc. collaborates with CSL Seqirus for mRNA technology to support the development and production of vaccines for SARS-CoV-2 (COVID-19), influenza, and prevalent respiratory viral infections.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 7.02 billion |
| Revenue Forecast by 2030 | USD 15.13 billion |
| Growth rate from 2022 to 2030 | CAGR of 8.91% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Vaccine type, Indication, Age group, Route of Administration, Distribution channel, Region |
| Companies Covered |
GlaxoSmithKline Plc, Biodiem, Sanofi, AstraZeneca, CSL Limited, Emergent Biosolutions, BIKEN Co, Ltd., F. Hoffmann-La Roche Ltd., Abbott Laboratories, Merck & Co., Inc., Sinovac Biotech Ltd., Novartis Ag, Viatris Inc., and Pfizer Inc. |
Quadrivalent vaccines essentially combat two Influenza-A viruses and two Influenza-B viruses, therefore they are most preferred for vaccination regimes. Companies are working significantly towards carrying out research studies that involve developing novel quadrivalent vaccines. For instance, in September 2022, Pfizer Inc. declared the start of its pivotal phase-3 clinical trial study for the evaluation of attributes like the safety and efficacy of its quadrivalent modified RNA (modRNA) candidate with over 25,000 healthy participants after achieving promising results from the first two phases.
Oral vaccines are preferred over vaccine injections because of the flexibility it offers for production, storage conditions, logistics, and administration. The oral forms of vaccinations may also potentially increase the rate of administration to the mass population. Research efforts are underway for devising novel oral vaccines that are equally efficient to injectable vaccines. For instance, in July 2019, Vaxart, Inc., collaborated with Janssen Vaccines B.V. for the evaluation of Vaxart’s oral vaccine platform with a potential candidate that is used in the form of a room temperature-stable tablet for Janssen’s universal influenza vaccination program.
Due to the emergence of virus strains, researchers are working towards devising a vaccination strategy that could combat multiple strains of the influenza virus and achieve long-lasting immunity in an individual. For instance, in March 2022, Researchers at the National University of Singapore and Monash University, Melbourne collaboratively published research in the ‘Proceedings of the National Academy of Sciences’ featuring a platform to effectively deliver vaccine candidate M2e to the immune cells so as to achieve long-lasting immunity against multiple strains of the virus.
Moreover, in May 2022, Osivax, signed a collaboration agreement with the National Institute of Allergy and Infectious Diseases (part of the NIH), to carry out a preclinical evaluation integrating Osivax’s T-cell-based influenza candidate, with a broad spectrum influenza vaccine candidates of the NIAID Research Center.
Measuring the vaccine’s potency and efficacy is crucial to gauge the immunity it offers against infection and is ascertained by the random administration of potential vaccine candidates to healthy volunteers through numerous clinical trials at different stages. For instance, in September 2022, the RAIVEN (Randomized Assessment of Influenza Vaccine Efficacy Network) was instated. RAIVEN is a collaborative effort between the Centre for Disease Control in the U.S. states for carrying out randomized trials.
Many countries across the globe are investing consistent efforts to achieve autonomy in vaccine production to rule out dependency on other countries for vaccine supply. Vaccines are most efficiently designed by research efforts by various research groups across research institutes and pharmaceutical giants. For instance, in May 2022, Blue Water Vaccines, Inc. partnered with the Center for R&D in Immunobiologics (CeRDI), Brazil for the development of a universal influenza vaccine candidate, so as to advance vaccine development based on novel adjuvant formulations and cell-cultures.
Key Players
Market Segmentation
By Vaccine type
By Indication
By Age group
By Route of Administration
By Distribution channel
By Region
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Influenza Vaccine Market
5.1. COVID-19 Landscape: Influenza Vaccine Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Influenza Vaccine Market, By Vaccine type
8.1. Influenza Vaccine Market, by Vaccine type, 2022-2030
8.1.1. Inactivated
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Live Attenuated
8.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Influenza Vaccine Market, By Indication
9.1. Influenza Vaccine Market, by Indication, 2022-2030
9.1.1. Quadrivalent
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Trivalent
9.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Influenza Vaccine Market, By Age group
10.1. Influenza Vaccine Market, by Age group, 2022-2030
10.1.1. Paediatric
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Adult
10.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Influenza Vaccine Market, By Route of Administration
11.1. Influenza Vaccine Market, by Route of Administration, 2022-2030
11.1.1. Injection
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Nasal Spray
11.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global Influenza Vaccine Market, By Distribution channel
12.1. Influenza Vaccine Market, by Distribution channel, 2022-2030
12.1.1. Hospitals & Pharmacy
12.1.1.1. Market Revenue and Forecast (2017-2030)
12.1.2. Government & Institutional supply
12.1.2.1. Market Revenue and Forecast (2017-2030)
12.1.3. Other
12.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 13. Global Influenza Vaccine Market, Regional Estimates and Trend Forecast
13.1. North America
13.1.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.1.2. Market Revenue and Forecast, by Indication (2017-2030)
13.1.3. Market Revenue and Forecast, by Age group (2017-2030)
13.1.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.1.5. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.1.6. U.S.
13.1.6.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.1.6.2. Market Revenue and Forecast, by Indication (2017-2030)
13.1.6.3. Market Revenue and Forecast, by Age group (2017-2030)
13.1.6.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.1.7. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.1.8. Rest of North America
13.1.8.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.1.8.2. Market Revenue and Forecast, by Indication (2017-2030)
13.1.8.3. Market Revenue and Forecast, by Age group (2017-2030)
13.1.8.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.1.8.5. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.2. Europe
13.2.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.2.2. Market Revenue and Forecast, by Indication (2017-2030)
13.2.3. Market Revenue and Forecast, by Age group (2017-2030)
13.2.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.2.5. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.2.6. UK
13.2.6.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.2.6.2. Market Revenue and Forecast, by Indication (2017-2030)
13.2.6.3. Market Revenue and Forecast, by Age group (2017-2030)
13.2.7. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.2.8. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.2.9. Germany
13.2.9.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.2.9.2. Market Revenue and Forecast, by Indication (2017-2030)
13.2.9.3. Market Revenue and Forecast, by Age group (2017-2030)
13.2.10. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.2.11. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.2.12. France
13.2.12.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.2.12.2. Market Revenue and Forecast, by Indication (2017-2030)
13.2.12.3. Market Revenue and Forecast, by Age group (2017-2030)
13.2.12.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.2.13. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.2.14. Rest of Europe
13.2.14.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.2.14.2. Market Revenue and Forecast, by Indication (2017-2030)
13.2.14.3. Market Revenue and Forecast, by Age group (2017-2030)
13.2.14.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.2.15. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.3. APAC
13.3.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.3.2. Market Revenue and Forecast, by Indication (2017-2030)
13.3.3. Market Revenue and Forecast, by Age group (2017-2030)
13.3.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.3.5. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.3.6. India
13.3.6.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.3.6.2. Market Revenue and Forecast, by Indication (2017-2030)
13.3.6.3. Market Revenue and Forecast, by Age group (2017-2030)
13.3.6.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.3.7. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.3.8. China
13.3.8.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.3.8.2. Market Revenue and Forecast, by Indication (2017-2030)
13.3.8.3. Market Revenue and Forecast, by Age group (2017-2030)
13.3.8.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.3.9. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.3.10. Japan
13.3.10.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.3.10.2. Market Revenue and Forecast, by Indication (2017-2030)
13.3.10.3. Market Revenue and Forecast, by Age group (2017-2030)
13.3.10.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.3.10.5. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.3.11. Rest of APAC
13.3.11.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.3.11.2. Market Revenue and Forecast, by Indication (2017-2030)
13.3.11.3. Market Revenue and Forecast, by Age group (2017-2030)
13.3.11.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.3.11.5. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.4. MEA
13.4.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.4.2. Market Revenue and Forecast, by Indication (2017-2030)
13.4.3. Market Revenue and Forecast, by Age group (2017-2030)
13.4.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.4.5. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.4.6. GCC
13.4.6.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.4.6.2. Market Revenue and Forecast, by Indication (2017-2030)
13.4.6.3. Market Revenue and Forecast, by Age group (2017-2030)
13.4.6.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.4.7. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.4.8. North Africa
13.4.8.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.4.8.2. Market Revenue and Forecast, by Indication (2017-2030)
13.4.8.3. Market Revenue and Forecast, by Age group (2017-2030)
13.4.8.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.4.9. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.4.10. South Africa
13.4.10.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.4.10.2. Market Revenue and Forecast, by Indication (2017-2030)
13.4.10.3. Market Revenue and Forecast, by Age group (2017-2030)
13.4.10.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.4.10.5. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.4.11. Rest of MEA
13.4.11.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.4.11.2. Market Revenue and Forecast, by Indication (2017-2030)
13.4.11.3. Market Revenue and Forecast, by Age group (2017-2030)
13.4.11.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.4.11.5. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.5. Latin America
13.5.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.5.2. Market Revenue and Forecast, by Indication (2017-2030)
13.5.3. Market Revenue and Forecast, by Age group (2017-2030)
13.5.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.5.5. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.5.6. Brazil
13.5.6.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.5.6.2. Market Revenue and Forecast, by Indication (2017-2030)
13.5.6.3. Market Revenue and Forecast, by Age group (2017-2030)
13.5.6.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.5.7. Market Revenue and Forecast, by Distribution channel (2017-2030)
13.5.8. Rest of LATAM
13.5.8.1. Market Revenue and Forecast, by Vaccine type (2017-2030)
13.5.8.2. Market Revenue and Forecast, by Indication (2017-2030)
13.5.8.3. Market Revenue and Forecast, by Age group (2017-2030)
13.5.8.4. Market Revenue and Forecast, by Route of Administration (2017-2030)
13.5.8.5. Market Revenue and Forecast, by Distribution channel (2017-2030)
Chapter 14. Company Profiles
14.1. GlaxoSmithKline Plc
14.1.1. Company Overview
14.1.2. Product Offerings
14.1.3. Financial Performance
14.1.4. Recent Initiatives
14.2. Biodiem
14.2.1. Company Overview
14.2.2. Product Offerings
14.2.3. Financial Performance
14.2.4. Recent Initiatives
14.3. Sanofi
14.3.1. Company Overview
14.3.2. Product Offerings
14.3.3. Financial Performance
14.3.4. Recent Initiatives
14.4. AstraZeneca
14.4.1. Company Overview
14.4.2. Product Offerings
14.4.3. Financial Performance
14.4.4. Recent Initiatives
14.5. CSL Limited
14.5.1. Company Overview
14.5.2. Product Offerings
14.5.3. Financial Performance
14.5.4. Recent Initiatives
14.6. Emergent Biosolutions
14.6.1. Company Overview
14.6.2. Product Offerings
14.6.3. Financial Performance
14.6.4. Recent Initiatives
14.7. BIKEN Co, Ltd.
14.7.1. Company Overview
14.7.2. Product Offerings
14.7.3. Financial Performance
14.7.4. Recent Initiatives
14.8. F. Hoffmann-La Roche Ltd.
14.8.1. Company Overview
14.8.2. Product Offerings
14.8.3. Financial Performance
14.8.4. Recent Initiatives
14.9. Abbott Laboratories
14.9.1. Company Overview
14.9.2. Product Offerings
14.9.3. Financial Performance
14.9.4. Recent Initiatives
14.10. Merck & Co., Inc.
14.10.1. Company Overview
14.10.2. Product Offerings
14.10.3. Financial Performance
14.10.4. Recent Initiatives
Chapter 15. Research Methodology
15.1. Primary Research
15.2. Secondary Research
15.3. Assumptions
Chapter 16. Appendix
16.1. About Us
16.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others