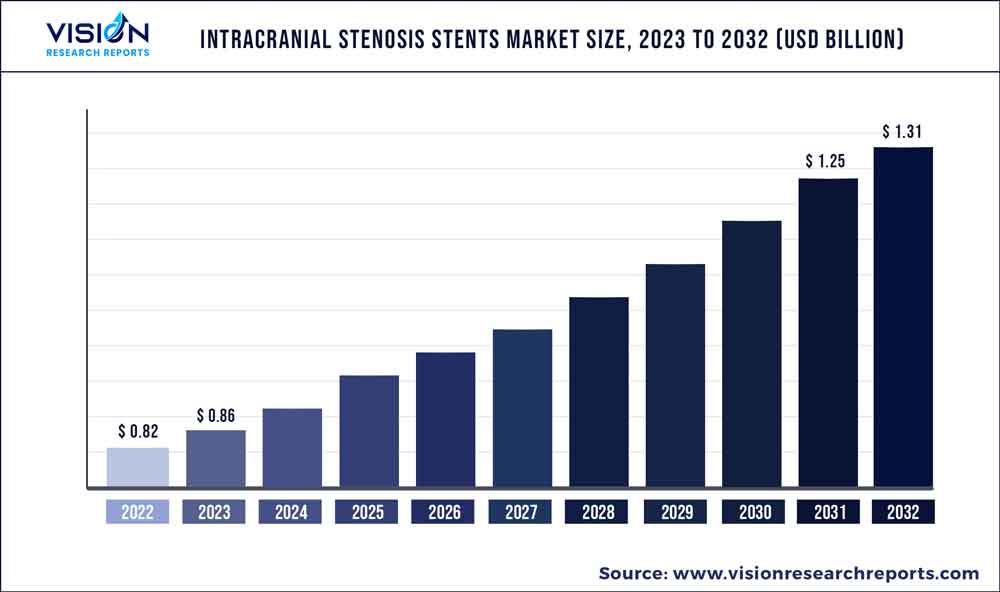
The global intracranial stenosis stents market was valued at USD 0.82 billion in 2022 and it is predicted to surpass around USD 1.31 billion by 2032 with a CAGR of 4.83% from 2023 to 2032. The intracranial stenosis stents market in the United States was accounted for USD 88 million in 2022.
Key Pointers
Report Scope of the Intracranial Stenosis Stents Market
| Report Coverage | Details |
| Revenue Share of North America in 2022 | 37% |
| Revenue Forecast by 2032 | USD 1.31 billion |
| Growth Rate from 2023 to 2032 | CAGR of 4.83% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Companies Covered | phenox GmbH; Medtronic; Acandis GmbH; MicroPort Scientific Corporation; MicroVention, Inc.; CERENOVUS (Johnson & Johnson); Sino Medical Sciences Technology, Inc.; Stryker; Balt |
The market is growing due to factors such as the rising incidence of stroke, increasing demand for minimally invasive intracranial surgeries, and the constantly growing geriatric population. For instance, according to a report from the World Stroke Organization from 2022, there are more than 12.2 million new stroke cases recorded annually worldwide. In addition, according to the same source, stroke is the most prevalent condition in people aged 70 years and older (67%), followed by those between the ages of 15 and 49 (22%). This factor is expected to fuel market growth over the coming years.
Minimally invasive surgeries are gaining popularity due to the reduced risk and trauma associated with these procedures. Smaller incisions decrease postoperative pain and facilitate speedy recovery, leading to the high adoption of these procedures. Several key players are heavily investing in R&D activities for the launch of innovative minimally invasive surgical instruments.
Cerebrovascular complications related to intracranial stenting refer to adverse events that can occur during or after the procedure, which affects the blood vessels and blood flow in the brain. The major cerebrovascular complications after an intracranial stenting procedure may be linked to low-volume locations, posterior circulation stenosis, and the stenting procedure itself.
For instance, according to a study published in the Journal of Stroke and Cerebrovascular Diseases, balloon-expandable intracranial stent placement can produce satisfactory outcomes in 97% of stable patients and 67% of unstable patients. Within the first 30 days following the procedure, 10% of cerebral stents were associated with adverse events, which included fatalities, one from a myocardial infarction (4%) mild stroke, and three major stroke cases.
The shortage of skilled professionals in intracranial stenosis stenting is a significant concern in many countries globally. Intracranial stenosis stenting is a highly specialized procedure that requires extensive training and experience to be performed safely & effectively. The shortage of trained professionals can limit access to this potentially life-saving treatment for patients, which restricts the growth of the intracranial stenosis stents market.
In the U.S., a shortage of neuro-interventionalists has been reported in some regions. This can limit access to specialized treatments such as intracranial stenosis stenting for patients with neurological conditions. A survey of U.S. healthcare providers published in the Journal of Stroke and Cerebrovascular Diseases in 2018 stated that only 40% of respondents reported feeling comfortable performing intracranial stenting procedures. Moreover, concerns about patient safety, lack of access to training programs, and limited reimbursement were identified as major barriers to developing expertise in this area.
The COVID-19 pandemic is estimated to have had a modest impact on the market in the short term. As the pandemic gradually came under control and the healthcare sector began to recover, the demand for intracranial stenosis stents increased. In addition, technological advancements in stent design and materials are driving growth in the market. For instance, in August 2020, Stryker received U.S. FDA approval for an expanded indication of its Neuroform Atlas Stent System. Such strategic initiatives are expected to drive the market during the forecast period.
Product Insights
Based on product, the self-expanding stents segment dominated the market with 35% of the revenue share in 2022. Intracranial stenosis is associated with the risk of ischemic stroke; however, research has shown that Self-Expandable Stents (SES) are likely to be safe for flow restoration in cases of acute ischemic strokes.
For instance, according to the CDC, stroke is a major cause of long-term disability. In addition, it reduces mobility in more than half of stroke survivors aged 65 and above. Therefore, intracranial stenosis stents are designed to prevent heart-attack occurrence through a self-expanding feature, where the deployed stent expands and holds the vessel open.
The growth of the embolization coil support intracranial stents segment is driven by the rising incidence of brain strokes, increasing target population, advancements in technology, improvements in healthcare facilities, and adoption of new surgical options. Due to these factors, the usage of stent-assisted coiling will continue to increase, thus leading to the growth of the segment. The use of embolization coil support intracranial stents has been one of the key options for treating intracranial stenosis.
End-use Insights
The hospital end-use segment dominated with 81% of the market share, which can be attributed to the rising prevalence of stenosis, aneurysm, brain stroke, and neurovascular diseases. The increasing demand for products such as self-expanding stents, venous sinus stents, and balloon-expanding & other stents in neurosurgery has led to increased sales of products. Moreover, the adoption of new technology-based devices in hospitals is a key factor for market growth. Alternatively, the rising number of neurosurgeries performed in hospitals has further led to the growth of the market.
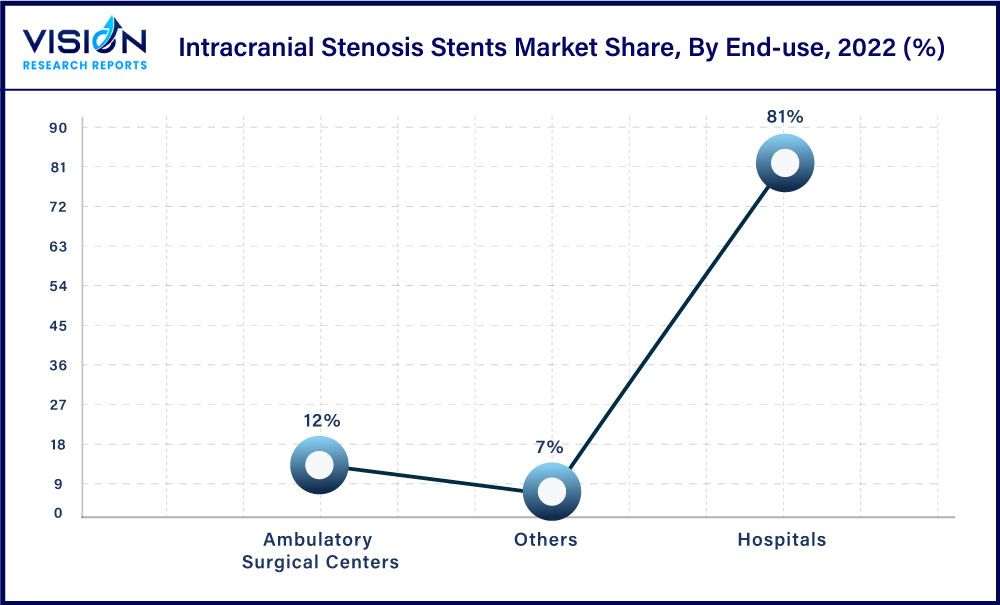
The ambulatory surgical center segment is expected to witness growth at a rapid pace over the estimated period. Ambulatory surgery centers are focusing on renovating offering in healthcare delivery with different facilities for brain-related injuries as well as new growth opportunities for medical devices and equipment. Ambulatory surgical centers offer low-risk procedures in a more convenient setting at lower rates than hospitals.
Regional Insights
North America accounted for the largest share of 37% in 2022. The regional growth is driven by the high incidence rates of hypertension & stroke and the presence of well-established healthcare facilities. According to the CDC, in 2020, approximately 670,000 deaths in the U.S. had hypertension as the primary contributing cause. Moreover, as per CDC, around 87% of strokes are ischemic strokes, where blood flow to the brain is blocked. Increasing demand for minimally invasive procedures and the rising prevalence of intracranial stenosis cases are expected to fuel market growth over the coming years.
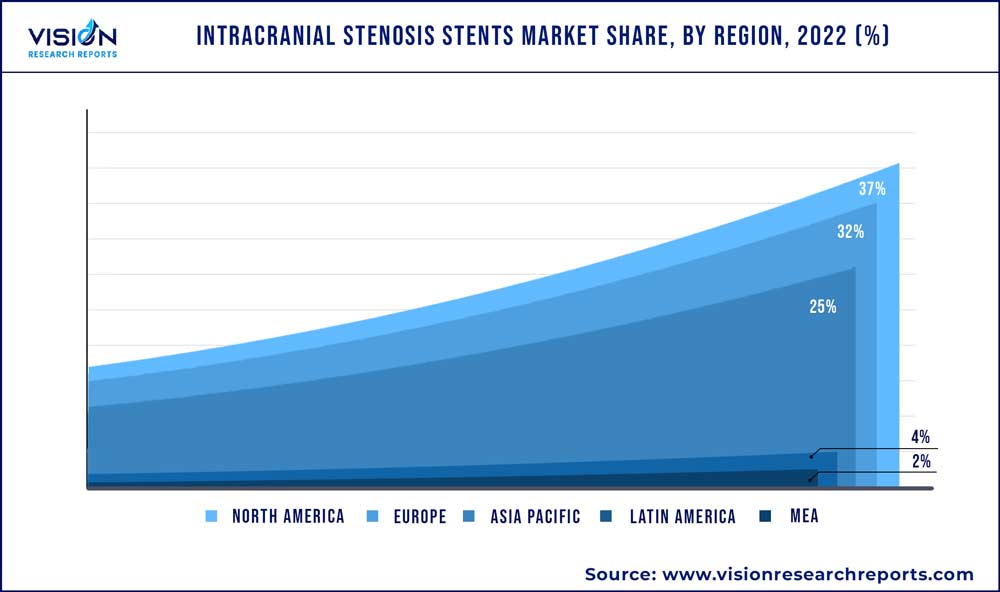
The Asia Pacific market for intracranial stenosis stents is expected to grow at the fastest rate of 5.73% over the forecast period. This can be attributed to factors such as the growing aging population, negative lifestyle choices & stress, rising incidence of ischemic stroke, large population, and increasing patient affordability in the region. Moreover, lucrative growth opportunities in developing economies, such as India and China, are likely to contribute to regional market growth.
Major global players are contributing to the market growth in the region by receiving approvals from authorities or expanding their product portfolios. For instance, in December 2020, MicroPort Scientific Corporation received approval from China’s National Medical Products Administration (NMPA) for its Bridge Vertebral Drug-Eluting Stent to treat symptomatic vertebral artery stenosis in China.
Intracranial Stenosis Stents Market Segmentations:
By Product
By End-use
By Regional
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Intracranial Stenosis Stents Market
5.1. COVID-19 Landscape: Intracranial Stenosis Stents Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Intracranial Stenosis Stents Market, By Product
8.1. Intracranial Stenosis Stents Market, by Product, 2023-2032
8.1.1. Self-expanding Stents
8.1.1.1. Market Revenue and Forecast (2020-2032)
8.1.2. Embolization Coil Support Intracranial Stents
8.1.2.1. Market Revenue and Forecast (2020-2032)
8.1.3. Venous Sinus Stents
8.1.3.1. Market Revenue and Forecast (2020-2032)
8.1.4. Balloon-expanding & Other Stents
8.1.4.1. Market Revenue and Forecast (2020-2032)
Chapter 9. Global Intracranial Stenosis Stents Market, By End-use
9.1. Intracranial Stenosis Stents Market, by End-use, 2023-2032
9.1.1. Hospitals
9.1.1.1. Market Revenue and Forecast (2020-2032)
9.1.2. Ambulatory Surgical Centers
9.1.2.1. Market Revenue and Forecast (2020-2032)
9.1.3. Others
9.1.3.1. Market Revenue and Forecast (2020-2032)
Chapter 10. Global Intracranial Stenosis Stents Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Product (2020-2032)
10.1.2. Market Revenue and Forecast, by End-use (2020-2032)
10.1.3. U.S.
10.1.3.1. Market Revenue and Forecast, by Product (2020-2032)
10.1.3.2. Market Revenue and Forecast, by End-use (2020-2032)
10.1.4. Rest of North America
10.1.4.1. Market Revenue and Forecast, by Product (2020-2032)
10.1.4.2. Market Revenue and Forecast, by End-use (2020-2032)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Product (2020-2032)
10.2.2. Market Revenue and Forecast, by End-use (2020-2032)
10.2.3. UK
10.2.3.1. Market Revenue and Forecast, by Product (2020-2032)
10.2.3.2. Market Revenue and Forecast, by End-use (2020-2032)
10.2.4. Germany
10.2.4.1. Market Revenue and Forecast, by Product (2020-2032)
10.2.4.2. Market Revenue and Forecast, by End-use (2020-2032)
10.2.5. France
10.2.5.1. Market Revenue and Forecast, by Product (2020-2032)
10.2.5.2. Market Revenue and Forecast, by End-use (2020-2032)
10.2.6. Rest of Europe
10.2.6.1. Market Revenue and Forecast, by Product (2020-2032)
10.2.6.2. Market Revenue and Forecast, by End-use (2020-2032)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Product (2020-2032)
10.3.2. Market Revenue and Forecast, by End-use (2020-2032)
10.3.3. India
10.3.3.1. Market Revenue and Forecast, by Product (2020-2032)
10.3.3.2. Market Revenue and Forecast, by End-use (2020-2032)
10.3.4. China
10.3.4.1. Market Revenue and Forecast, by Product (2020-2032)
10.3.4.2. Market Revenue and Forecast, by End-use (2020-2032)
10.3.5. Japan
10.3.5.1. Market Revenue and Forecast, by Product (2020-2032)
10.3.5.2. Market Revenue and Forecast, by End-use (2020-2032)
10.3.6. Rest of APAC
10.3.6.1. Market Revenue and Forecast, by Product (2020-2032)
10.3.6.2. Market Revenue and Forecast, by End-use (2020-2032)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Product (2020-2032)
10.4.2. Market Revenue and Forecast, by End-use (2020-2032)
10.4.3. GCC
10.4.3.1. Market Revenue and Forecast, by Product (2020-2032)
10.4.3.2. Market Revenue and Forecast, by End-use (2020-2032)
10.4.4. North Africa
10.4.4.1. Market Revenue and Forecast, by Product (2020-2032)
10.4.4.2. Market Revenue and Forecast, by End-use (2020-2032)
10.4.5. South Africa
10.4.5.1. Market Revenue and Forecast, by Product (2020-2032)
10.4.5.2. Market Revenue and Forecast, by End-use (2020-2032)
10.4.6. Rest of MEA
10.4.6.1. Market Revenue and Forecast, by Product (2020-2032)
10.4.6.2. Market Revenue and Forecast, by End-use (2020-2032)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Product (2020-2032)
10.5.2. Market Revenue and Forecast, by End-use (2020-2032)
10.5.3. Brazil
10.5.3.1. Market Revenue and Forecast, by Product (2020-2032)
10.5.3.2. Market Revenue and Forecast, by End-use (2020-2032)
10.5.4. Rest of LATAM
10.5.4.1. Market Revenue and Forecast, by Product (2020-2032)
10.5.4.2. Market Revenue and Forecast, by End-use (2020-2032)
Chapter 11. Company Profiles
11.1. phenox GmbH
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Medtronic
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Acandis GmbH
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. MicroPort Scientific Corporation
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. LTE Scientific
11.5. MicroVention, Inc.
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. CERENOVUS (Johnson & Johnson)
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Sino Medical Sciences Technology, Inc.
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Stryker
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Balt
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others