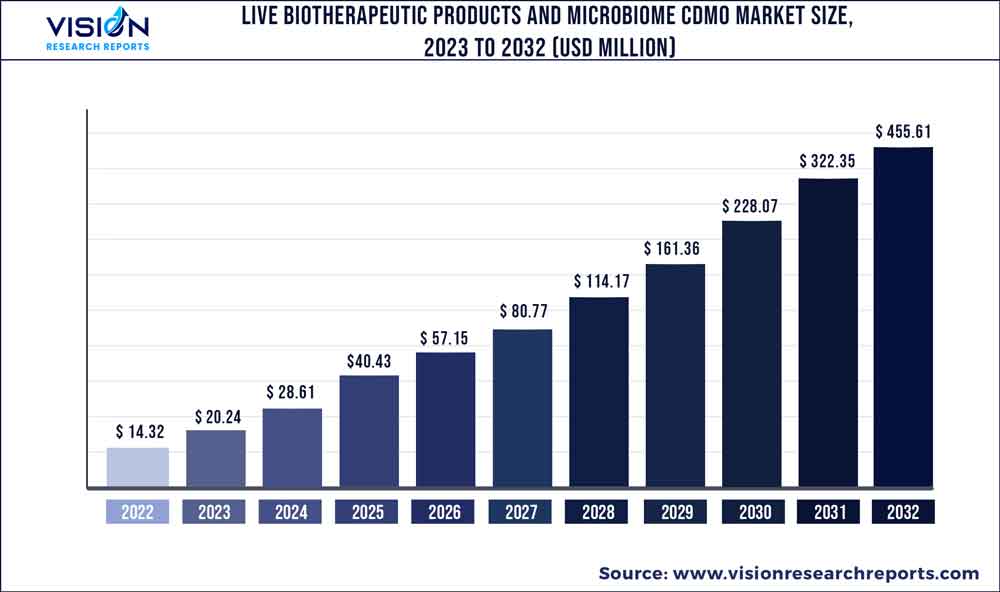
The global live biotherapeutics products and microbe CDMO market was surpassed at USD 14.32 million in 2022 and is expected to hit around USD 455.61 million by 2032, growing at a CAGR of 41.34% from 2023 to 2032. By application segment, the live biotherapeutics products and microbe CDMO market in the United States was accounted for USD 10.3 million in 2022.
Key Pointers
Report Scope of the Live Biotherapeutic Products And Microbiome CDMO Market
| Report Coverage | Details |
| Revenue Share of North America in 2022 | 26.27% |
| Revenue Forecast by 2032 | USD 455.61 million |
| Growth rate from 2023 to 2032 | CAGR of 41.34% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Companies Covered | Arrant Bio; 4D Pharma; Cerbios; Biose Industrie; Assembly Bioscience Inc.; Wacker Chemie AG; Quay Pharmaceuticals; NIZO; Lonza; Inpac Probiotics |
The market for microbiome CDMOs and Live Biotherapeutic Products (LBPs) has been growing quickly in recent years, and this trend is anticipated to continue. the rising understanding of the potential advantages of live biotherapeutic products and microbiome-based therapeutics in treating a variety of diseases, including inflammatory bowel disease, cancer, and neurological disorders, among patients and healthcare providers.
The need for CDMOs specializing in these fields is being driven by the expanding investment by pharmaceutical and biotech businesses in live biotherapeutic products and microbiome-based medicines, and this trend is anticipated to continue in the next ten years. Companies are looking for CDMOs with the skills required to develop and produce these products as the market for LBPs and microbiome-based therapeutics grows.This has resulted in the growth of the global CDMO market, with significant expansion expected in the coming years. Outsourcing to CDMOs is becoming an attractive option for pharmaceutical and biotech companies due to the increasing complexity of developing these therapies.
Applications-based market segments have been created for live biotherapeutic products and microbiome-based therapeutics, with the C. difficile category currently dominating the market. Effective medicines are desperately required to tackle the major and expanding health issue of recurrent C. difficile infection. For instance, SER-109, a microbiome therapy created by Seres Therapeutics for the treatment of recurrent C. difficile infection, has demonstrated good outcomes in clinical trials. The FDA has given the business SER-109's breakthrough therapy classification; if approved, this would position the company as a significant contender in the market for C. difficile medicines. The market for live biotherapeutic products and microbiome-based medicines is anticipated to develop since there is an increasing need for effective treatments for C. difficile and other illnesses.
The COVID-19 pandemic has had a significant impact on the market for live biotherapeutic products and microbiome Contract Development and Manufacturing Organizations (CDMOs). The disruption caused by the pandemic has led to delays in clinical trials, decreased demand for certain products, and supply chain disruptions. However, with the easing of pandemic-related restrictions and the increasing focus on healthcare, the market for LBPs and microbiome-based therapies is expected to rebound in the coming years. Companies in this space will need to adapt to new challenges and find innovative solutions to maintain growth in a post-pandemic world.
Application Insights
The C.difficle dominated the market and held 83.63% of the market share in 2022. On the basis of application, the live biotherapeutic products and microbiome CDMO industry is segmented into C.difficle, Crohns disease, IBS, Diabetes, and Others. C. difficile is a bacterium that is found in the human gut microbiome and can cause severe diarrhea and colitis, particularly in people who have recently taken antibiotics. In recent years, there has been growing interest in using live biotherapeutic products (LBPs) to treat a range of diseases, including those related to the gut microbiome.
Live biotherapeutics products are living organisms, such as bacteria or viruses, that are used to prevent or treat diseases. C. difficile is one of the organisms that can be used as an LBP, and there has been some research into its potential use for treating C. difficile infections. As research into the human microbiome and the potential applications of LBPs continues to grow, it is likely that the market will show more interest in the use of C. difficile and other organisms for therapeutic purposes.
Regional Insights
North America held dominant share of 79.25% in 2022, primarily due to the presence of key players and increasing investments in research and development activities in this region. The growing demand for effective treatments for various diseases such as C.difficile, Crohn's disease, and IBS, has also contributed to the market growth in North America.
Moreover, the region has a large number of established pharmaceutical and biotech companies with expertise in the development and commercialization of biologic products, including live biotherapeutic products and microbiome-based therapies. Additionally, the region has a well-established regulatory framework for biopharmaceuticals, which provides a stable environment for companies to develop and manufacture these products. Adding to it, North America has a strong research infrastructure, including top universities and research institutions, that drive innovation in the field. This has led to the development of cutting-edge technologies and techniques that are used in the production of live biotherapeutic products and microbiome-based therapies.
Live Biotherapeutic Products And Microbiome CDMO Market Segmentations:
By Application
By Regional
Chapter 1. Introduction
1.1.Research Objective
1.2.Scope of the Study
1.3.Definition
Chapter 2. Research Methodology
2.1.Research Approach
2.2.Data Sources
2.3.Assumptions & Limitations
Chapter 3. Executive Summary
3.1.Market Snapshot
Chapter 4. Market Variables and Scope
4.1.Introduction
4.2.Market Classification and Scope
4.3.Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5.COVID 19 Impact on Live Biotherapeutic Products And Microbiome CDMO Market
5.1. COVID-19 Landscape: Live Biotherapeutic Products And Microbiome CDMO Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4.Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1.Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2.Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1.List of Suppliers
7.1.3.2.List of Buyers
Chapter 8. Global Live Biotherapeutic Products And Microbiome CDMO Market, By Application
8.1.Live Biotherapeutic Products And Microbiome CDMO Market, by Application Type, 2023-2032
8.1.1. C.difficle
8.1.1.1.Market Revenue and Forecast (2020-2032)
8.1.2. Crohns Disease
8.1.2.1.Market Revenue and Forecast (2020-2032)
8.1.3. IBS
8.1.3.1.Market Revenue and Forecast (2020-2032)
8.1.4. Diabetes
8.1.4.1.Market Revenue and Forecast (2020-2032)
8.1.5. Others
8.1.5.1.Market Revenue and Forecast (2020-2032)
Chapter 9. Global Live Biotherapeutic Products And Microbiome CDMO Market, Regional Estimates and Trend Forecast
9.1. North America
9.1.1. Market Revenue and Forecast, by Application (2020-2032)
9.1.2. U.S.
9.1.2.1. Market Revenue and Forecast, by Application (2020-2032)
9.1.3. Rest of North America
9.1.3.1. Market Revenue and Forecast, by Application (2020-2032)
9.2. Europe
9.2.1. Market Revenue and Forecast, by Application (2020-2032)
9.2.2. UK
9.2.2.1. Market Revenue and Forecast, by Application (2020-2032)
9.2.3. Germany
9.2.3.1. Market Revenue and Forecast, by Application (2020-2032)
9.2.4. France
9.2.4.1. Market Revenue and Forecast, by Application (2020-2032)
9.2.5. Rest of Europe
9.2.5.1. Market Revenue and Forecast, by Application (2020-2032)
9.3. APAC
9.3.1. Market Revenue and Forecast, by Application (2020-2032)
9.3.2. India
9.3.2.1. Market Revenue and Forecast, by Application (2020-2032)
9.3.3. China
9.3.3.1. Market Revenue and Forecast, by Application (2020-2032)
9.3.4. Japan
9.3.4.1. Market Revenue and Forecast, by Application (2020-2032)
9.3.5. Rest of APAC
9.3.5.1. Market Revenue and Forecast, by Application (2020-2032)
9.4. MEA
9.4.1. Market Revenue and Forecast, by Application (2020-2032)
9.4.2. GCC
9.4.2.1. Market Revenue and Forecast, by Application (2020-2032)
9.4.3. North Africa
9.4.3.1. Market Revenue and Forecast, by Application (2020-2032)
9.4.4. South Africa
9.4.4.1. Market Revenue and Forecast, by Application (2020-2032)
9.4.5. Rest of MEA
9.4.5.1. Market Revenue and Forecast, by Application (2020-2032)
9.5. Latin America
9.5.1. Market Revenue and Forecast, by Application (2020-2032)
9.5.2. Brazil
9.5.2.1. Market Revenue and Forecast, by Application (2020-2032)
9.5.3. Rest of LATAM
9.5.3.1. Market Revenue and Forecast, by Application (2020-2032)
Chapter 10.Company Profiles
10.1. Arrant Bio
10.1.1.Company Overview
10.1.2.Product Offerings
10.1.3.Financial Performance
10.1.4.Recent Initiatives
10.2. 4D Pharma
10.2.1.Company Overview
10.2.2.Product Offerings
10.2.3.Financial Performance
10.2.4.Recent Initiatives
10.3. Cerbios
10.3.1.Company Overview
10.3.2.Product Offerings
10.3.3.Financial Performance
10.3.4.Recent Initiatives
10.4. Biose Industrie
10.4.1.Company Overview
10.4.2.Product Offerings
10.4.3.Financial Performance
10.4.4.Recent Initiatives
10.5. Assembly Bioscience Inc.
10.5.1.Company Overview
10.5.2.Product Offerings
10.5.3.Financial Performance
10.5.4.Recent Initiatives
10.6. Wacker Chemie AG
10.6.1.Company Overview
10.6.2.Product Offerings
10.6.3.Financial Performance
10.6.4.Recent Initiatives
10.7. Quay Pharmaceuticals
10.7.1.Company Overview
10.7.2.Product Offerings
10.7.3.Financial Performance
10.7.4.Recent Initiatives
10.8. NIZO
10.8.1.Company Overview
10.8.2.Product Offerings
10.8.3.Financial Performance
10.8.4.Recent Initiatives
10.9. Lonza
10.9.1.Company Overview
10.9.2.Product Offerings
10.9.3.Financial Performance
10.9.4.Recent Initiatives
10.10. Inpac Probiotics
10.10.1. Company Overview
10.10.2. Product Offerings
10.10.3. Financial Performance
10.10.4. Recent Initiatives
Chapter 11.Research Methodology
11.1.Primary Research
11.2.Secondary Research
11.3.Assumptions
Chapter 12.Appendix
12.1. About Us
12.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others