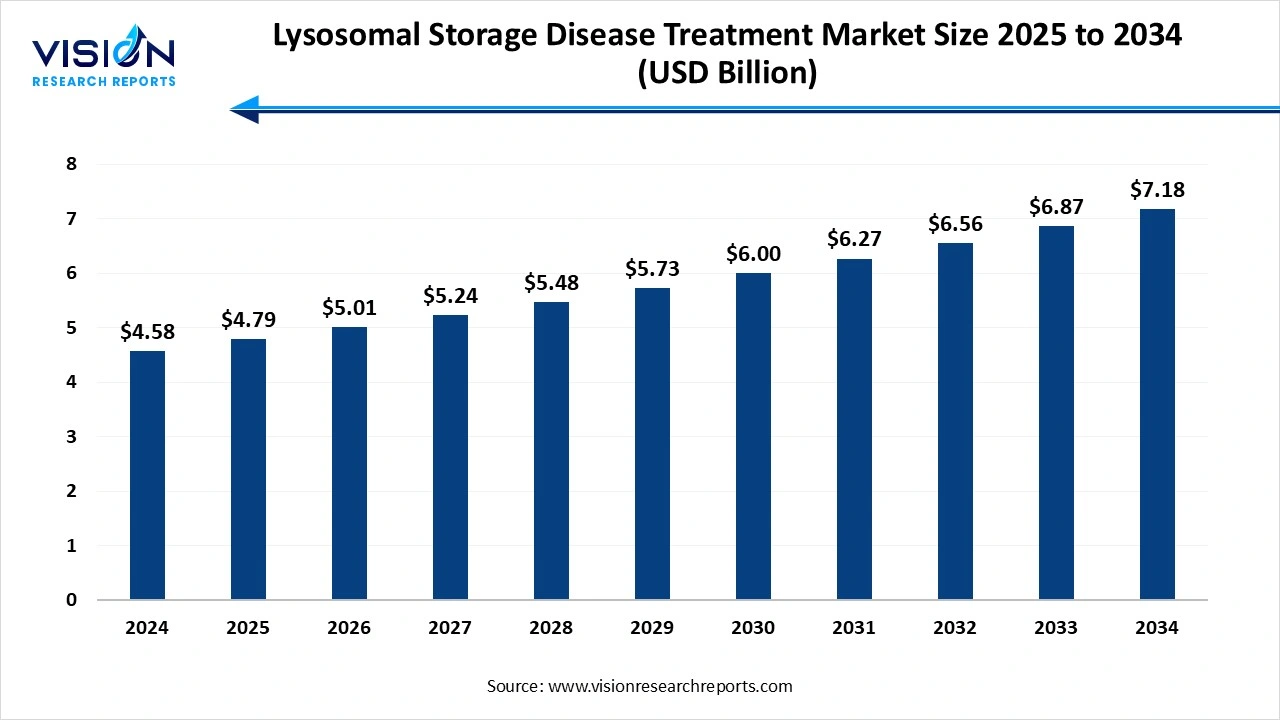
The global lysosomal storage disease treatment market size was estimated at USD 4.58 billion in 2024, expected to grow to USD 4.79 billion in 2025, and projected to exceed USD 7.18 billion by 2034, with a CAGR ranging from 4.6% over the forecast period.The market growth is driven by increasing awareness of rare genetic disorders, the Lysosomal Storage Disease Treatment Market is experiencing growth due to advancements in enzyme replacement therapies and gene therapy innovations.
Lysosomal storage diseases or disorders (LSDs) are rare genetic conditions that cause a buildup of toxic materials in your body’s cells. People with LSDs lack certain substances that helps the enzymes work. Without functioning enzymes, your body is unable to break down fats and sugars. If those build up in your body, they can be quite harmful. Lysosomal storage diseases usually appear during pregnancy or soon after birth.
The lysosomal storage disease (LSD) treatment market is experiencing steady growth, driven by increasing awareness, advancements in enzyme replacement therapies (ERT) and rising diagnostic capabilities. Although these disorders are rare, the growing global prevalence due to improved screening techniques has created a significant demand for innovative treatments.
The lysosomal storage disease (LSD) treatment market is primarily driven by advancements in therapeutic technologies such as enzyme replacement therapy (ERT), gene therapy, and substrate reduction therapy. These innovations have significantly improved the management of various LSDs, offering longer life expectancy and better quality of life for patients. Additionally, the increased availability of genetic testing and newborn screening programs has led to earlier diagnosis, allowing for prompt and more effective treatment interventions.
Regulatory support through orphan drug incentives, fast-track approvals, and growing awareness among healthcare providers and patients are further accelerating market growth. The expansion of healthcare infrastructure and increasing investments in rare disease research, especially in emerging economies, are also contributing to market expansion.
Increasing Prevalence of LSDs
The increasing incidence of lysosomal storage diseases (LSDs) on a global level is a key driver in the Lysosomal Storage Diseases Treatment Market. With an estimated prevalence of almost 1 in 7,700 births for certain LSDs, the demand for effective therapies is always on the rise. This situation is particularly evident in regions that have improved diagnostic capabilities, thus leading to earlier detection and treatment. As awareness about childcare and genetics grows, healthcare systems are increasingly prioritizing the management and treatment of such rare diseases, expanding the market’s potential.
Another major key driver is the advancement of newborn screening programs across the world, which has led to earlier diagnosis of various LSDs. Countries are progressively expanding their recommended panels to include conditions such as Pompe disease and MPS I. Early detection enables the timely initiation of treatment, significantly improving long-term disease outcomes. As more and more countries implement such measures, this will enhance proactive management and align industry strategies with early therapeutic interventions.
Complications in Diagnosis
Lysosomal storage diseases (LSDs) are rare genetic disorders that present significant diagnostic challenges. Their symptoms are often non-specific, and their severity varies widely among individuals, making it difficult for healthcare providers to accurately diagnose the condition without specialized testing.
Further complicating diagnosis is the lack of well-defined biomarkers and the variability in the age of symptom onset. With multiple causative genes involved, genetic testing becomes complex, and access to specialized diagnostic tools remains limited in certain regions and healthcare settings.
Patient Advocacy Groups and Regulatory Agencies
Patient advocacy groups and healthcare organizations have also contributed to raising public awareness, creating key opportunities for the market and encouraging individuals to seek medical attention, thus increasing diagnosis rates. Efforts to develop novel therapeutic approaches including gene therapy, substrate reduction therapy and small molecule drugs are ongoing. Innovative treatments like enzyme replacement therapy and gene editing technologies further contribute to the expansive range of available options for managing and treating lysosomal storage diseases.
Regulatory agencies are another such key opportunity. These agencies are increasingly supportive of the development of therapies for rare diseases, including lysosomal storage disorders. Initiatives such as orphan drug designations and detailed review processes help to encourage pharmaceutical companies in investing in this niche market. This regulatory environment fosters innovation and boosts the availability of new treatments, thus leading to market growth and development.
North America accounted for the largest share of the lysosomal storage disease (LSDs) treatment market in 2024, capturing 37% of the total market. This is because the region benefits from favorable regulatory frameworks such as orphan drug incentives, which support the development and commercialization of therapies for rare diseases like LSDs. Additionally, the availability of advanced treatment options, comprehensive insurance coverage and active patient advocacy networks contribute to the region’s market dominance. Additionally, the region is home to major pharmaceutical companies that continue to actively invest in clinical research and novel enzyme therapies. High awareness among physicians and patients also supports the rapid adoption of such treatments..
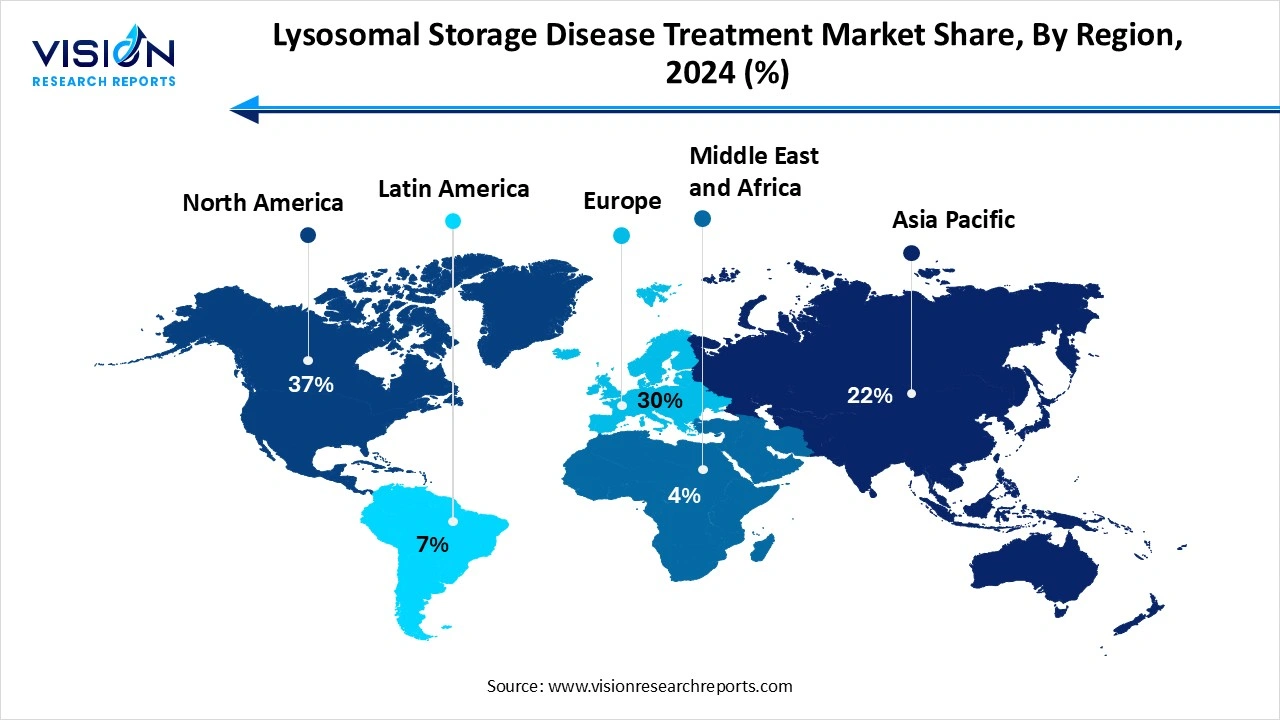
Asia Pacific is expected to witness the fastest growth rate during the forecast period. The region’s rapid growth is fueled by rising healthcare investments, growing awareness of rare diseases and the gradual improvement in diagnostic and therapeutic infrastructure. Countries all across the region are improving the process of rare disease detection by adopting advanced genetic testing technologies. Pharmaceutical firms are also entering into strategic alliances in order to boost therapy availability and scale distribution. Rising healthcare expenditure and urbanization are also contributing to better access to specialist care.
Which treatment type held the largest market share in 2024?
The enzyme replacement therapy segment led the market, accounting for the largest revenue share of 84% in 2024. This type of treatment works by delivering a functional version of the missing or defective enzyme to the patient's body, helping to break down the accumulated substrates within cells. This leads to significantly improved clinical outcomes for various LSDs, such as Gaucher disease, Fabry disease and Pompe disease. Despite its high cost, ERT continues to be widely adopted, especially in developed markets where reimbursement policies and healthcare infrastructure support its use.
The substrate reduction therapy segment is anticipated to experience the fastest growth. This therapy works by limiting the production of substrates that accumulate due to enzyme deficiencies, thus reducing the burden on the lysosomal system. This oral treatment option is gaining popularity due to its ease of administration and potential to improve patient compliance.
Which disease type dominated the market in 2024?
The fabry disease held the largest revenue share in 2024, accounting for 30% of the market. This type of disease is caused by a deficiency of the enzyme alpha-galactosidase A, leading to the accumulation of globotriaosylceramide in blood vessels, kidneys, heart and nervous system. The disease affects both males and females, although symptoms tend to be more severe in males. Over the years, increased awareness and improved diagnostic capabilities have led to early detection and timely intervention.
The Pompe disease segment is expected to witness rapid growth. This disorder can present itself at any time, whether in infancy or adulthood, with infantile-onset Pompe disease being more severe and rapidly progressive. The availability of enzyme replacement therapy has significantly improved survival rates and quality of life, especially when treatment begins early.
By Treatment Type
By Disease Type
By Regional
Lysosomal Storage Disease Treatment Market
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Lysosomal Storage Disease Treatment Market
5.1. COVID-19 Landscape: Lysosomal Storage Disease Treatment Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Lysosomal Storage Disease Treatment Market, By Treatment Type
8.1. Lysosomal Storage Disease Treatment Market, by Treatment Type, 2024-2034
8.1.1. Enzyme Replacement Therapy
8.1.1.1. Market Revenue and Forecast (2024-2034)
8.1.2. Substrate Reduction Therapy
8.1.2.1. Market Revenue and Forecast (2024-2034)
8.1.3. Others
8.1.3.1. Market Revenue and Forecast (2024-2034)
Chapter 9. Global Lysosomal Storage Disease Treatment Market, By Disease Type
9.1. Lysosomal Storage Disease Treatment Market, by Disease Type, 2024-2034
9.1.1. Gaucher Disease
9.1.1.1. Market Revenue and Forecast (2024-2034)
9.1.2. Mucopolysaccharidoses
9.1.2.1. Market Revenue and Forecast (2024-2034)
9.1.3. Pompe Disease
9.1.3.1. Market Revenue and Forecast (2024-2034)
9.1.4. Fabry Disease
9.1.4.1. Market Revenue and Forecast (2024-2034)
9.1.5. Others
9.1.5.1. Market Revenue and Forecast (2024-2034)
Chapter 10. Global Lysosomal Storage Disease Treatment Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Treatment Type
11.1.2. Market Revenue and Forecast, by Disease Type
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Treatment Type
11.1.4.2. Market Revenue and Forecast, by Disease Type
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Treatment Type
11.1.5.2. Market Revenue and Forecast, by Disease Type
11.2. Europe
11.2.1. Market Revenue and Forecast, by Treatment Type
11.2.2. Market Revenue and Forecast, by Disease Type
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Treatment Type
11.2.4.2. Market Revenue and Forecast, by Disease Type
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Treatment Type
11.2.5.2. Market Revenue and Forecast, by Disease Type
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Treatment Type
11.2.6.2. Market Revenue and Forecast, by Disease Type
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Treatment Type
11.2.7.2. Market Revenue and Forecast, by Disease Type
11.3. APAC
11.3.1. Market Revenue and Forecast, by Treatment Type
11.3.2. Market Revenue and Forecast, by Disease Type
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Treatment Type
11.3.4.2. Market Revenue and Forecast, by Disease Type
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Treatment Type
11.3.5.2. Market Revenue and Forecast, by Disease Type
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Treatment Type
11.3.6.2. Market Revenue and Forecast, by Disease Type
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Treatment Type
11.3.7.2. Market Revenue and Forecast, by Disease Type
11.4. MEA
11.4.1. Market Revenue and Forecast, by Treatment Type
11.4.2. Market Revenue and Forecast, by Disease Type
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Treatment Type
11.4.4.2. Market Revenue and Forecast, by Disease Type
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Treatment Type
11.4.5.2. Market Revenue and Forecast, by Disease Type
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Treatment Type
11.4.6.2. Market Revenue and Forecast, by Disease Type
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Treatment Type
11.4.7.2. Market Revenue and Forecast, by Disease Type
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Treatment Type
11.5.2. Market Revenue and Forecast, by Disease Type
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Treatment Type
11.5.4.2. Market Revenue and Forecast, by Disease Type
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Treatment Type
11.5.5.2. Market Revenue and Forecast, by Disease Type
Chapter 11. Company Profiles
11.1. Takeda Pharmaceutical Company Limited
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. BioMarin Pharmaceutical Inc.
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Amicus Therapeutics, Inc.
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. Pfizer Inc.
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. Johnson & Johnson
11.5. Intermountain Life Sciences
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Alexion Pharmaceuticals, Inc. (AstraZeneca)
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Avrobio, Inc.
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Actelion Pharmaceuticals Ltd
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Protalix BioTherapeutics, Inc.
11.9. Evoqua Water Technologies
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others