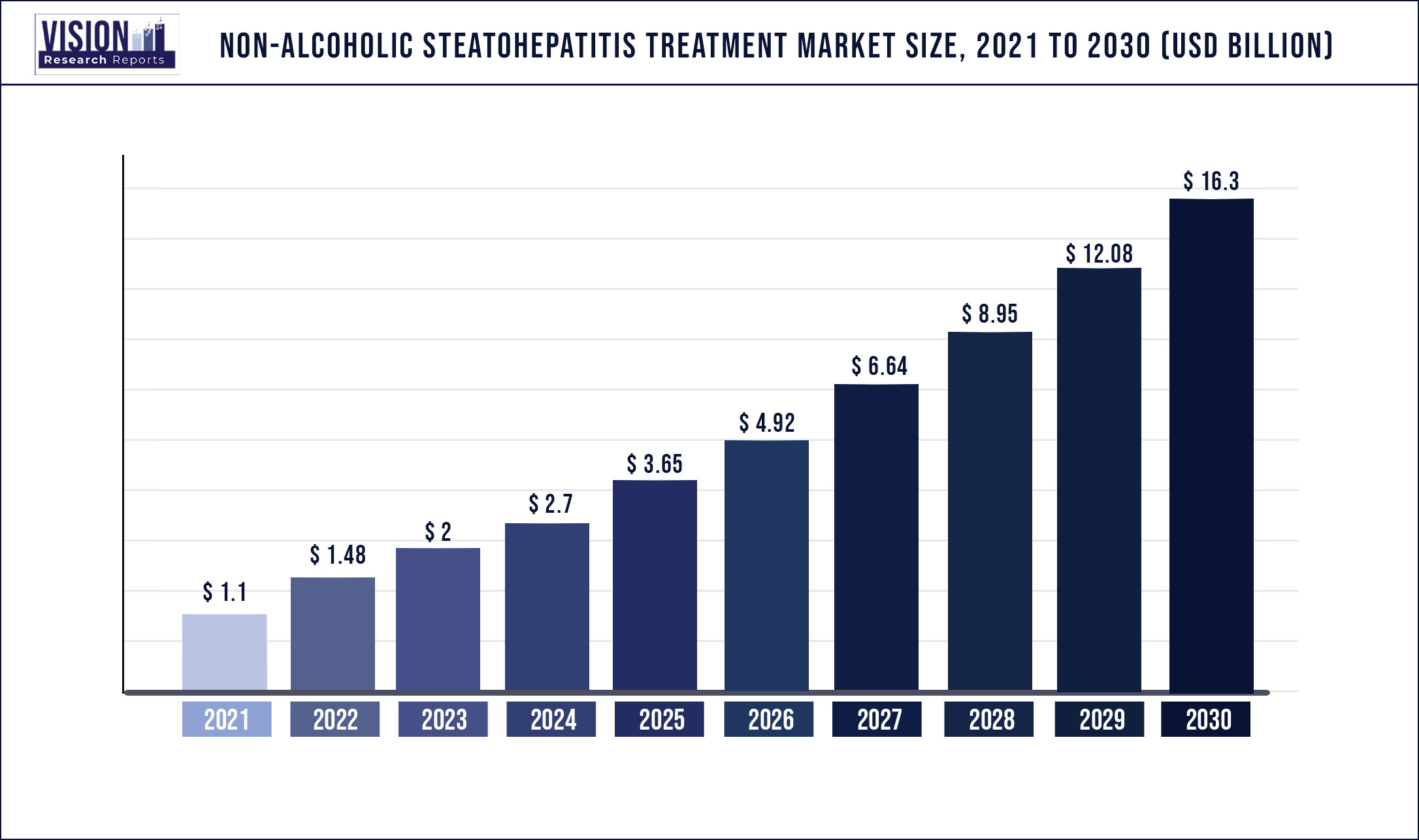
The global non-alcoholic steatohepatitis (NASH) treatment market was valued at USD 1.1 billion in 2021 and it is predicted to surpass around USD 16.3 billion by 2030 with a CAGR of 34.92% from 2022 to 2030
The non-alcoholic steatohepatitis (NASH) treatment market is primarily driven by the launch of drugs such as Novo Nordisk's Ozempic, Intercept's Ocaliva, and Inventiva's lanifibranor, among others.
The NASH treatment market has major unmet needs owing to factors such as the unavailability of approved drugs, high disease burden, and complex diagnostic procedures. Currently, the market is dominated by off-label drugs such as Pioglitazone and Vitamin E. These are widely prescribed drugs in this space globally. However, to address the unmet opportunity, key market players are heavily investing in R&D activities to develop novel therapeutics for NASH treatment. Currently, there are more than 50 pipeline candidates.
Some of the late-stage pipeline candidates expected to launch during the forecast period include Inventiva Pharma's Lanifibranor, Intercept Pharmaceuticals' Obeticholic acid, Galmed Pharmaceuticals Ltd.'s Aramchol, Novo Nordisk A/S's Semaglutide, and Madrigal Pharmaceuticals, Inc.'s Resmetirom. Among these, Intercept Pharmaceuticals's Obeticholic Acid (OCA), and Madrigal Pharmaceuticals, Inc.'s Resmetirom, are the most looked upon drugs and are expected to enter comparatively earlier than other pipeline candidates.
Research studies reveal that non-alcoholic steatohepatitis is strongly associated with obesity and diabetes. Research studies show that around 80% of NASH patients are obese. In countries such as the U.S., the obesity prevalence is as high as 42%, according to the latest 2021 statistics by CDC. In addition, countries such as the U.S. and Japan account for the highest prevalence of non-alcoholic steatohepatitis globally, and these countries also have a high burden of obesity and diabetes. All such factors will fuel the NASH treatment market throughout the forecast period.
Strategic initiatives by pharma giants and supportive regulatory authority policies such as fast track designation are further expected to accelerate the market growth. In May 2022, Pfizer, Inc.'s Ervogastat/Clesacostat Combination therapy received fast track designation. This is one of the potential drug combinations for the treatment of non-alcoholic steatohepatitis. Such drug designation boosts the clinical trial process for drugs.
Liver biopsy is the gold standard for the diagnosis of non-alcoholic steatohepatitis. The invasive nature of this diagnostics test limits its usage to only symptomatic cases, and as a result, the global average diagnostic rate for NASH is around 20%. The unavailability of biomarkers-based non-invasive tests for the diagnosis of non-alcoholic steatohepatitis is expected to impede the market growth.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 1.1 billion |
| Revenue Forecast by 2030 | USD 16.3 billion |
| Growth rate from 2022 to 2030 | CAGR of 34.92% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Drug type, end-use, region |
| Companies Covered | Intercept Pharmaceuticals; Galmed Pharmaceuticals; Inventiva Pharma; AbbVie Inc.; Galectin Therapeutics Inc.; Madrigal Pharmaceuticals; NGM Biopharmaceuticals Inc.; Novo Nordisk A/S; Bristol Myers Squibb; Gilead Sciences Inc. |
Drug Type Insights
Vitamin E and pioglitazone segment completely dominated the market with a share of 100% in 2021. The domination of off-label therapies can be attributed to the unavailability of approved therapeutic options in the market. Off-label treatments like Vitamin E are the most commonly prescribed therapeutics for non-alcoholic steatohepatitis.
However, Vitamin E is not recommended for diabetic patients due to the medical complications associated with it. In contrast, Pioglitazone is administered to NASH patients with type 2 diabetes. Some other off-label drugs include statins, which are prescribed to NASH patients with a risk of cardiovascular disease.
The NASH market is highly engaged in robust R&D activities; various potential pathways are in R&D for NASH treatments, including lipogenesis inhibitors, medications targeting the farnesoid X receptor axis, ASK1 inhibitors, and many more. Some prominent drugs anticipated to be launched during the forecast period include Intercept Pharmaceuticals' Obeticholic acid, Inventiva Pharma's Lanifibranor, Novo Nordisk A/S's Semaglutide, Galmed Pharmaceuticals Ltd's Aramchol, and Madrigal Pharmaceuticals, Inc.'s Resmetirom.
End-Use Insights
The retail and specialty pharmacies segment is anticipated to expand at a substantial CAGR of 39.6% during the forecast period. As NASH is a chronic disease, it takes a long period of support of medications, which favors retail pharmacies' growth. Most medication consumption, including off-label, is anticipated in homecare settings associated with retail pharmacies. All these factors are expected to propel the market growth during the assessment period.
The others segment is expected to progress at the highest CAGR of 42.07% during the forecast period. Other end-use segments include online pharmacy; this platform encourages ease of buying for customers. The trend of online purchasing is growing due to the platform's comfort, flexibility, and convenience, thus generating high revenues and driving the segment.
Regional Insights
In 2021, North America accounted for the largest market share of 79.62%. This can be attributed tothe high disease burden, increased healthcare expenditure, rise in patient awareness, and presence of major players in the region. In addition, the U.S. accounted for the highest prevalence of cases for NASH, followed by Japan.
Spain was the least affected among the European countries, and Germany had the highest NASH prevalence. In addition, the Asia Pacific region is expected to witness substantial growth during the forecast period. This is more likely due to high NASH prevalent countries such as Japan.
Key Players
Market Segmentation
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Non-Alcoholic Steatohepatitis Treatment Market
5.1. COVID-19 Landscape: Non-Alcoholic Steatohepatitis Treatment Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Non-Alcoholic Steatohepatitis Treatment Market, By Drug Type
8.1. Non-Alcoholic Steatohepatitis Treatment Market, by Drug Type, 2022-2030
8.1.1. Vitamin E and Pioglitazone
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Obeticholic Acid (OCA)
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Lanifibranor
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Semaglutide
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Resmetirom
8.1.5.1. Market Revenue and Forecast (2017-2030)
8.1.6. Aramchol
8.1.6.1. Market Revenue and Forecast (2017-2030)
8.1.7. Cenicriviroc
8.1.7.1. Market Revenue and Forecast (2017-2030)
8.1.8. Others
8.1.8.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Non-Alcoholic Steatohepatitis Treatment Market, By End-use
9.1. Non-Alcoholic Steatohepatitis Treatment Market, by End-use, 2022-2030
9.1.1. Hospital Pharmacies
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Retail & Specialty Pharmacies
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Others
9.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Non-Alcoholic Steatohepatitis Treatment Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.1.2. Market Revenue and Forecast, by End-use (2017-2030)
10.1.3. U.S.
10.1.3.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.1.3.2. Market Revenue and Forecast, by End-use (2017-2030)
10.1.4. Rest of North America
10.1.4.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.1.4.2. Market Revenue and Forecast, by End-use (2017-2030)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.2.2. Market Revenue and Forecast, by End-use (2017-2030)
10.2.3. UK
10.2.3.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.2.3.2. Market Revenue and Forecast, by End-use (2017-2030)
10.2.4. Germany
10.2.4.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.2.4.2. Market Revenue and Forecast, by End-use (2017-2030)
10.2.5. France
10.2.5.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.2.5.2. Market Revenue and Forecast, by End-use (2017-2030)
10.2.6. Rest of Europe
10.2.6.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.2.6.2. Market Revenue and Forecast, by End-use (2017-2030)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.3.2. Market Revenue and Forecast, by End-use (2017-2030)
10.3.3. India
10.3.3.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.3.3.2. Market Revenue and Forecast, by End-use (2017-2030)
10.3.4. China
10.3.4.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.3.4.2. Market Revenue and Forecast, by End-use (2017-2030)
10.3.5. Japan
10.3.5.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.3.5.2. Market Revenue and Forecast, by End-use (2017-2030)
10.3.6. Rest of APAC
10.3.6.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.3.6.2. Market Revenue and Forecast, by End-use (2017-2030)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.4.2. Market Revenue and Forecast, by End-use (2017-2030)
10.4.3. GCC
10.4.3.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.4.3.2. Market Revenue and Forecast, by End-use (2017-2030)
10.4.4. North Africa
10.4.4.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.4.4.2. Market Revenue and Forecast, by End-use (2017-2030)
10.4.5. South Africa
10.4.5.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.4.5.2. Market Revenue and Forecast, by End-use (2017-2030)
10.4.6. Rest of MEA
10.4.6.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.4.6.2. Market Revenue and Forecast, by End-use (2017-2030)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.5.2. Market Revenue and Forecast, by End-use (2017-2030)
10.5.3. Brazil
10.5.3.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.5.3.2. Market Revenue and Forecast, by End-use (2017-2030)
10.5.4. Rest of LATAM
10.5.4.1. Market Revenue and Forecast, by Drug Type (2017-2030)
10.5.4.2. Market Revenue and Forecast, by End-use (2017-2030)
Chapter 11. Company Profiles
11.1. Intercept Pharmaceuticals
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Galmed Pharmaceuticals
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Inventiva Pharma
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. AbbVie Inc.
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. LTE Scientific
11.5. Galectin Therapeutics Inc.
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Madrigal Pharmaceuticals
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. NGM Biopharmaceuticals Inc.
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Novo Nordisk A/S
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Bristol Myers Squibb
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. Gilead Sciences Inc.
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others