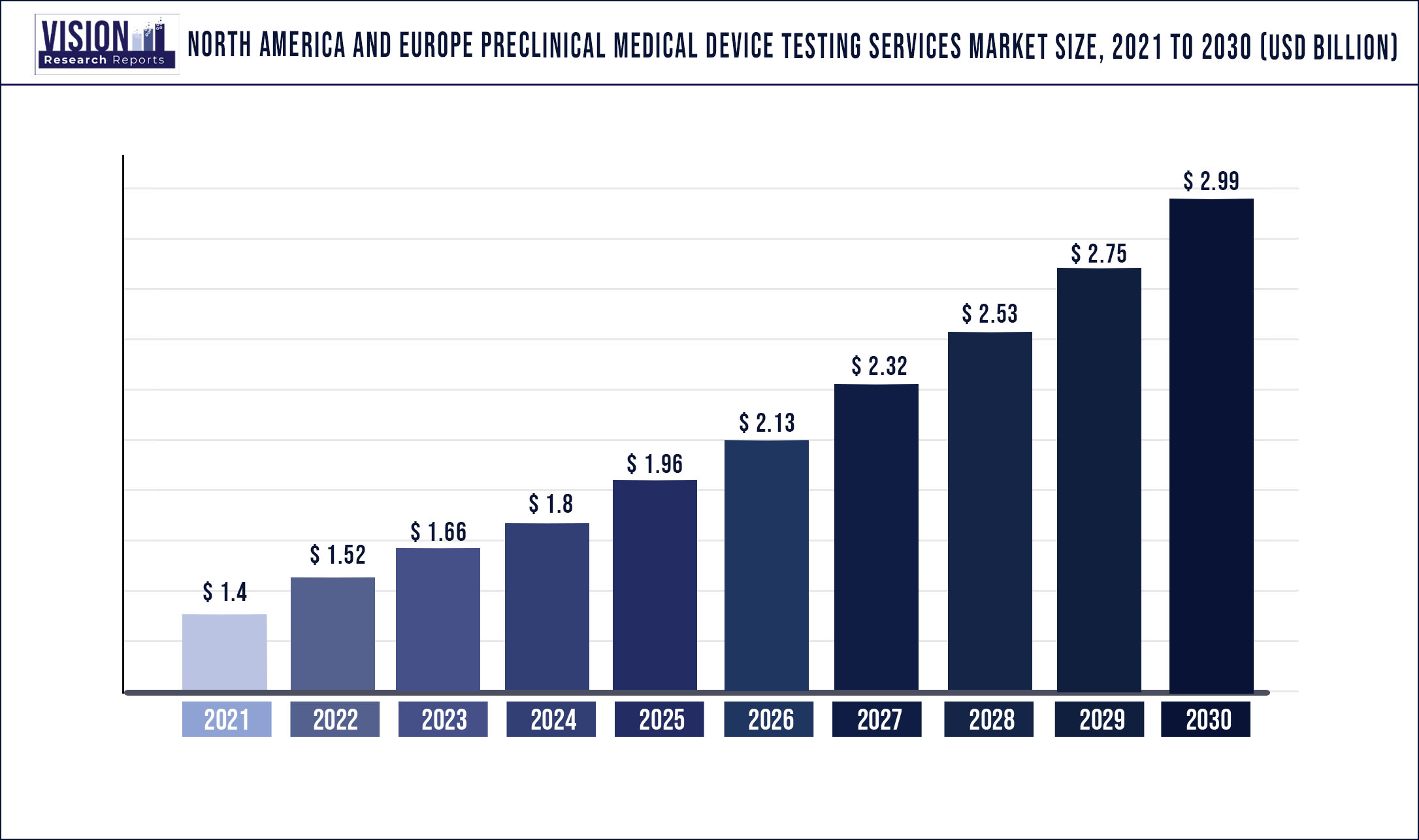
The North America and Europe preclinical medical device testing services market was surpassed at USD 1.4 billion in 2021 and is expected to hit around USD 2.99 billion by 2030, growing at a CAGR of 8.8% from 2022 to 2030
Report Highlights
The increase in the number of small medical devices lacking in-house testing capabilities and complexity in product design are the major factors driving the growth of the market.
There has been an increase in the number of players operating in the market over the last decade. Due to this large number, it has witnessed fierce competition. To sustain the market, a mix of defensive and offensive marketing strategies is used. For instance, extensive R&D, competitive pricing, new product launches, collaborative development, regional expansion, and mergers and acquisitions.
The COVID-19 pandemic has created a huge demand for these services. The rise was not significant in the first half, but it became more significant in the second as the industry adapted to operating during the pandemic. There has been an increase in the production and testing of personal protective equipment, and several projects that were put on hold because of COVID have resumed personal protective equipment. The epidemic has increased demand for a wide range of medical gadgets, diverting attention from those needed for surgery. COVID-19 vaccinations, ventilators, and pulse oximeters are the main goods seeing an increase in demand.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 1.4 billion |
| Revenue Forecast by 2030 | USD 2.99 billion |
| Growth rate from 2022 to 2030 | CAGR of 8.8% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Service, Region |
| Companies Covered | SGS SA, Toxikon, Inc.; Eurofins Scientific; Pace Analytical Services LLC; Intertek Group Plc; WUXI APPTEC; TÜV SÜD AG; Sterigenics International LLC; Nelson Labs; North American Science Associates, Inc.; American Preclinical Services; Charles River Laboratories International, Inc. |
Services Insights
The Microbiology & sterility testing segment accounted for the maximum revenue share of 34.4% in 2021. It is one of the major tests included in pre-clinical medical device testing. Manufacturing safe and effective goods is a top priority for medical device manufacturers, and sterility assurance is a crucial step in accomplishing this aim. By using radiation techniques like e-beam or gamma rays, many single-use medical devices are terminally sterilized. Additionally, confirmation of the sterilization procedure is required by the FDA and other regulatory agencies, which typically call for sterility testing.
The chemistry test segment is anticipated to exhibit the fastest CAGR of 8.96% during the forecast period. Medical devices are also subjected to chemistry tests, along with pharmaceutical formulations. Testing is performed to check if medical devices contain any solutes or chemicals that may leach into the surrounding environment when used with the intended liquid. These tests help investigate the safety parameters of medical devices. Hence, the FDA is also facilitating additional data analysis, such as polymer and colorant examinations, to provide a more holistic view of the safety of these tests. This is expected to positively impact the segment growth during the forecast period.
Regional Insights
North America led the market for preclinical medical device testing services, with the largest revenue share of 63.6% in 2021. This is largely due to the presence of a large number of players in this region. It is also the top manufacturing hub for complex, highly reliable, and high-end medical devices. There is a rapid increase in the manufacturing of medical devices to meet the rising demand for efficient and cost-effective healthcare in this region. Besides this, the presence of the FDA is fueling the growth of the market for medical device testing services.
In Europe, the preclinical medical device testing service is expected to witness a CAGR of 8.7% during the forecast period. This is due to the rising demand for cost-cutting and increasing complexity in product design, which has a high impact on the rendering drivers for the medical device analytical testing outsourcing market in European nations. A steady increase in the outsourcing of services for contract manufacturers and component suppliers has been observed in the last two decades. However, notified bodies such as the European Medical Device Regulation (EMDR) are also involved in the scrutiny of outsourced medical devices.
Key Players
Market Segmentation
Chapter 1. Introduction
1.1.Research Objective
1.2.Scope of the Study
1.3.Definition
Chapter 2. Research Methodology
2.1.Research Approach
2.2.Data Sources
2.3.Assumptions & Limitations
Chapter 3. Executive Summary
3.1.Market Snapshot
Chapter 4. Market Variables and Scope
4.1.Introduction
4.2.Market Classification and Scope
4.3.Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5.COVID 19 Impact on North America And Europe Preclinical Medical Device Testing Services Market
5.1. COVID-19 Landscape: North America And Europe Preclinical Medical Device Testing Services Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4.Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1.Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2.Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1.List of Suppliers
7.1.3.2.List of Buyers
Chapter 8. Global North America And Europe Preclinical Medical Device Testing Services Market, By Service
8.1.North America And Europe Preclinical Medical Device Testing Services Market, by Service Type, 2020-2027
8.1.1. Biocompatibility Tests
8.1.1.1.Market Revenue and Forecast (2016-2027)
8.1.2. Chemistry Test
8.1.2.1.Market Revenue and Forecast (2016-2027)
8.1.3. Microbiology & Sterility Testing
8.1.3.1.Market Revenue and Forecast (2016-2027)
8.1.4. Package Validation
8.1.4.1.Market Revenue and Forecast (2016-2027)
Chapter 9. Global North America And Europe Preclinical Medical Device Testing Services Market, Regional Estimates and Trend Forecast
9.1.North America
9.1.1. Market Revenue and Forecast, by Service (2016-2027)
9.1.2. U.S.
9.1.3. Rest of North America
9.1.3.1.Market Revenue and Forecast, by Service (2016-2027)
9.2.Europe
9.2.1. Market Revenue and Forecast, by Service (2016-2027)
9.2.2. UK
9.2.2.1.Market Revenue and Forecast, by Service (2016-2027)
9.2.3. France
9.2.3.1.Market Revenue and Forecast, by Service (2016-2027)
9.2.4. Rest of Europe
9.2.4.1.Market Revenue and Forecast, by Service (2016-2027)
Chapter 10.Company Profiles
10.1.SGS SA
10.1.1.Company Overview
10.1.2.Product Offerings
10.1.3.Financial Performance
10.1.4.Recent Initiatives
10.2.Toxikon, Inc.
10.2.1.Company Overview
10.2.2.Product Offerings
10.2.3.Financial Performance
10.2.4.Recent Initiatives
10.3.Eurofins Scientific
10.3.1.Company Overview
10.3.2.Product Offerings
10.3.3.Financial Performance
10.3.4.Recent Initiatives
10.4.Pace Analytical Services LLC
10.4.1.Company Overview
10.4.2.Product Offerings
10.4.3.Financial Performance
10.4.4.Recent Initiatives
10.5.Intertek Group Plc
10.5.1.Company Overview
10.5.2.Product Offerings
10.5.3.Financial Performance
10.5.4.Recent Initiatives
10.6.WUXI APPTEC
10.6.1.Company Overview
10.6.2.Product Offerings
10.6.3.Financial Performance
10.6.4.Recent Initiatives
10.7.TÜV SÜD AG
10.7.1.Company Overview
10.7.2.Product Offerings
10.7.3.Financial Performance
10.7.4.Recent Initiatives
10.8.Sterigenics International LLC
10.8.1.Company Overview
10.8.2.Product Offerings
10.8.3.Financial Performance
10.8.4.Recent Initiatives
10.9.Nelson Labs
10.9.1.Company Overview
10.9.2.Product Offerings
10.9.3.Financial Performance
10.9.4.Recent Initiatives
10.10.North American Science Associates, Inc.
10.10.1. Company Overview
10.10.2. Product Offerings
10.10.3. Financial Performance
10.10.4. Recent Initiatives
Chapter 11.Research Methodology
11.1.Primary Research
11.2.Secondary Research
11.3.Assumptions
Chapter 12.Appendix
12.1.About Us
12.2.Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others