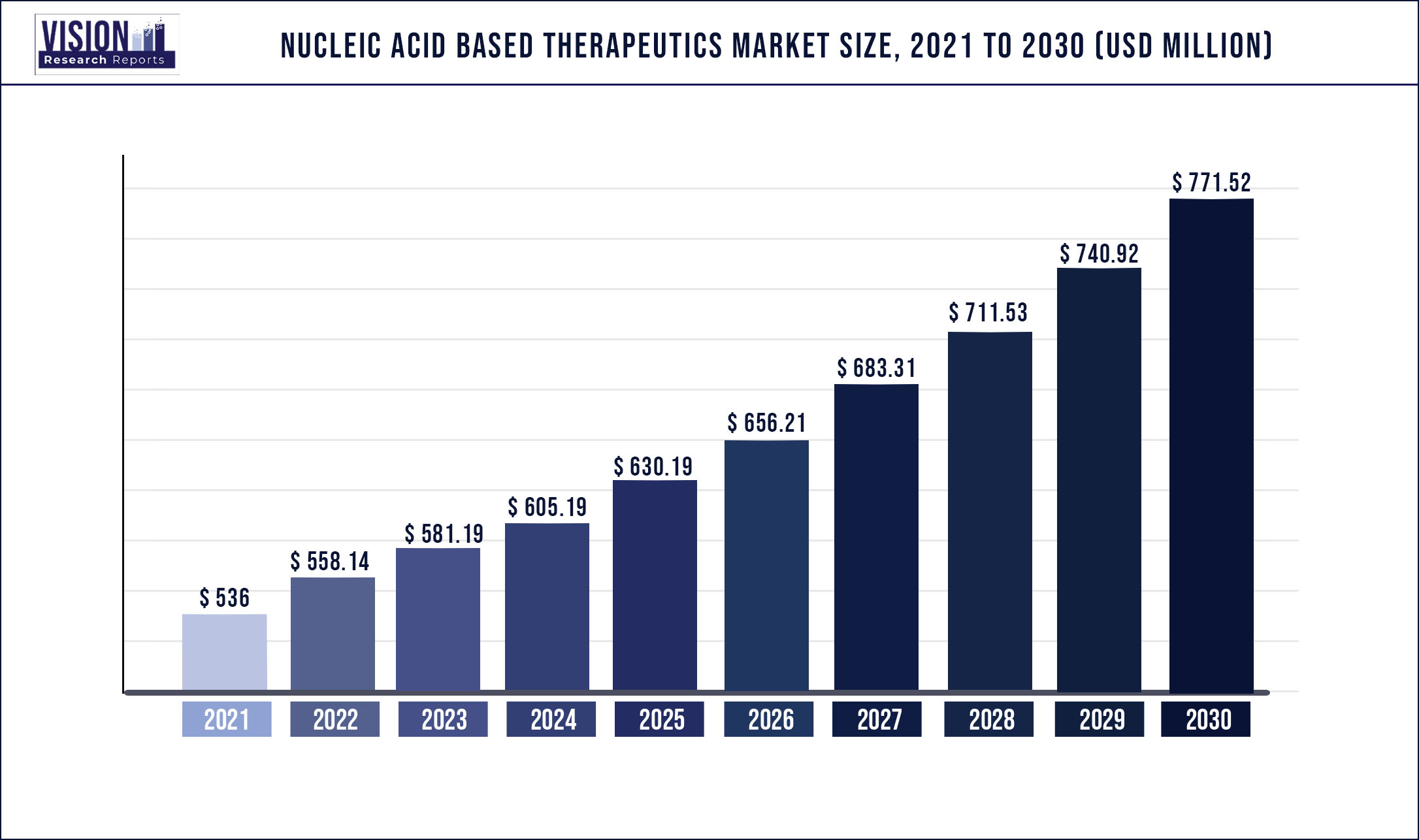
The global nucleic acids based therapeutics market was valued at USD 536 million in 2021 and it is predicted to surpass around USD 771.52 million by 2030 with a CAGR of 4.13% from 2022 to 2030.
Nucleic acid based therapeutics (NAT) target the genetic basis of the disease for treatment. Technological advancement and human genome understanding have allowed the development of many nucleic acids based therapeutics and even the approvals by US FDA and EMA demonstrates the potential to treat diseases genetically. NAT even allows long-lasting effects as it involves the cellular mechanism of gene silencing, addition, replacement, or editing.
The rising investments in the healthcare sector have also driven the research toward the treatment of many diseases and disorders. The increasing rate of chronic diseases among the population along with the genetic disorders has resulted in an upswing in research activities for building better healthcare settings worldwide. NAT offers a targeted and effective solution for any diseased condition. The COVID-19 pandemic also fueled the NAT market, as the successful utilization of mRNA vaccines as one of the preventive treatments boosted the healthcare industry to recognize the importance and efficacy of nucleic acid based therapies.
Nucleic acid based therapeutics market is segmented based on the following technologies;
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 536 million |
| Revenue Forecast by 2030 | USD 771.52 million |
| Growth rate from 2022 to 2030 | CAGR of 4.13% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Technology, Application, End-user, Region |
| Companies Covered |
Silence Therapeutics plc, Ionis Pharmaceuticals, Novartis, Arrowhead Pharmaceuticals, BioNtech, Moderna Inc., and Alnylam Pharmaceuticals, Inc. among others |
Many approved and late stage clinical programs are using RNA targeted treatment platform. Use of ASO (antisense oligonucleotides) or siRNA (short interfering RNA) for treatment of muscular atrophy has been approved by FDA and EMA. The widespread knowledge of the human genome and thus the advent of gene therapies for development & clinical use approval has boosted in the past years. The well-used examples of Kymriah, Yescarta, Tecartus for treatment of types of leukemia or lymphoma demonstrates the growing use of gene therapies. Novel identification of therapeutic targets in the non-coding genome are arising from the epigenetics research. The importance of genome editing techniques via CRISPR/Cas has been established in many applications such as stem cell. These gene editing therapeutics are the next generation of NAT market. Furthermore, other technologies such as LNPs (lipid nanoparticles), adeno-associated virus (AAV) vectors and many more also hold some significance in the NAT market.
The advancement in molecular technologies and the continuous omics-based research has resulted in huge investments by many companies. The nucleic acid based therapeutics market is highly competitive and the market participation is via many strategic business models. Acquisitions, partnerships or collaborations are the leading revenue generators in the market. For instance, in August 2022, Merck & Co. announced that it has collaborated with Orna Therapeutics to develop a new class of therapeutic RNA. The collaboration involved the discovery, development and commercialization of new RNA-based therapeutics for vaccines, infectious disease as well as oncology programs.
Key Players
Market Segmentation
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Nucleic Acid Based Therapeutics Market
5.1. COVID-19 Landscape: Nucleic Acid Based Therapeutics Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Nucleic Acid Based Therapeutics Market, By Technology
8.1. Nucleic Acid Based Therapeutics Market, by Technology, 2022-2030
8.1.1 RNA targeted therapeutics
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Gene therapies
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Epigenetic and microRNA modulating therapies
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Genome editing therapies
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Others
8.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Nucleic Acid Based Therapeutics Market, By Application
9.1. Nucleic Acid Based Therapeutics Market, by Application, 2022-2030
9.1.1. Autoimmune Disorders
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Infectious Diseases
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Genetic Disorders
9.1.3.1. Market Revenue and Forecast (2017-2030)
9.1.4. Cancer
9.1.4.1. Market Revenue and Forecast (2017-2030)
9.1.5. Others
9.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Nucleic Acid Based Therapeutics Market, By End-user
10.1. Nucleic Acid Based Therapeutics Market, by End-user, 2022-2030
10.1.1. Hospitals & Clinics
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Academic & Research Institutes
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Others
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Nucleic Acid Based Therapeutics Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Technology (2017-2030)
11.1.2. Market Revenue and Forecast, by Application (2017-2030)
11.1.3. Market Revenue and Forecast, by End-user (2017-2030)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Technology (2017-2030)
11.1.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.1.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Technology (2017-2030)
11.1.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.1.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Technology (2017-2030)
11.2.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Technology (2017-2030)
11.2.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Technology (2017-2030)
11.2.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Technology (2017-2030)
11.2.6.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.6.3. Market Revenue and Forecast, by End-user (2017-2030)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Technology (2017-2030)
11.2.7.2. Market Revenue and Forecast, by Application (2017-2030)
11.2.7.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Technology (2017-2030)
11.3.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Technology (2017-2030)
11.3.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Technology (2017-2030)
11.3.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Technology (2017-2030)
11.3.6.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.6.3. Market Revenue and Forecast, by End-user (2017-2030)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Technology (2017-2030)
11.3.7.2. Market Revenue and Forecast, by Application (2017-2030)
11.3.7.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Technology (2017-2030)
11.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Technology (2017-2030)
11.4.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Technology (2017-2030)
11.4.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Technology (2017-2030)
11.4.6.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.6.3. Market Revenue and Forecast, by End-user (2017-2030)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Technology (2017-2030)
11.4.7.2. Market Revenue and Forecast, by Application (2017-2030)
11.4.7.3. Market Revenue and Forecast, by End-user (2017-2030)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Technology (2017-2030)
11.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.5.3. Market Revenue and Forecast, by End-user (2017-2030)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Technology (2017-2030)
11.5.4.2. Market Revenue and Forecast, by Application (2017-2030)
11.5.4.3. Market Revenue and Forecast, by End-user (2017-2030)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Technology (2017-2030)
11.5.5.2. Market Revenue and Forecast, by Application (2017-2030)
11.5.5.3. Market Revenue and Forecast, by End-user (2017-2030)
Chapter 12. Company Profiles
12.1. Silence Therapeutics plc
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Ionis Pharmaceuticals
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Novartis
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Arrowhead Pharmaceuticals
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. BioNtech
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Moderna Inc.
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Alnylam Pharmaceuticals, Inc.
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. others
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others