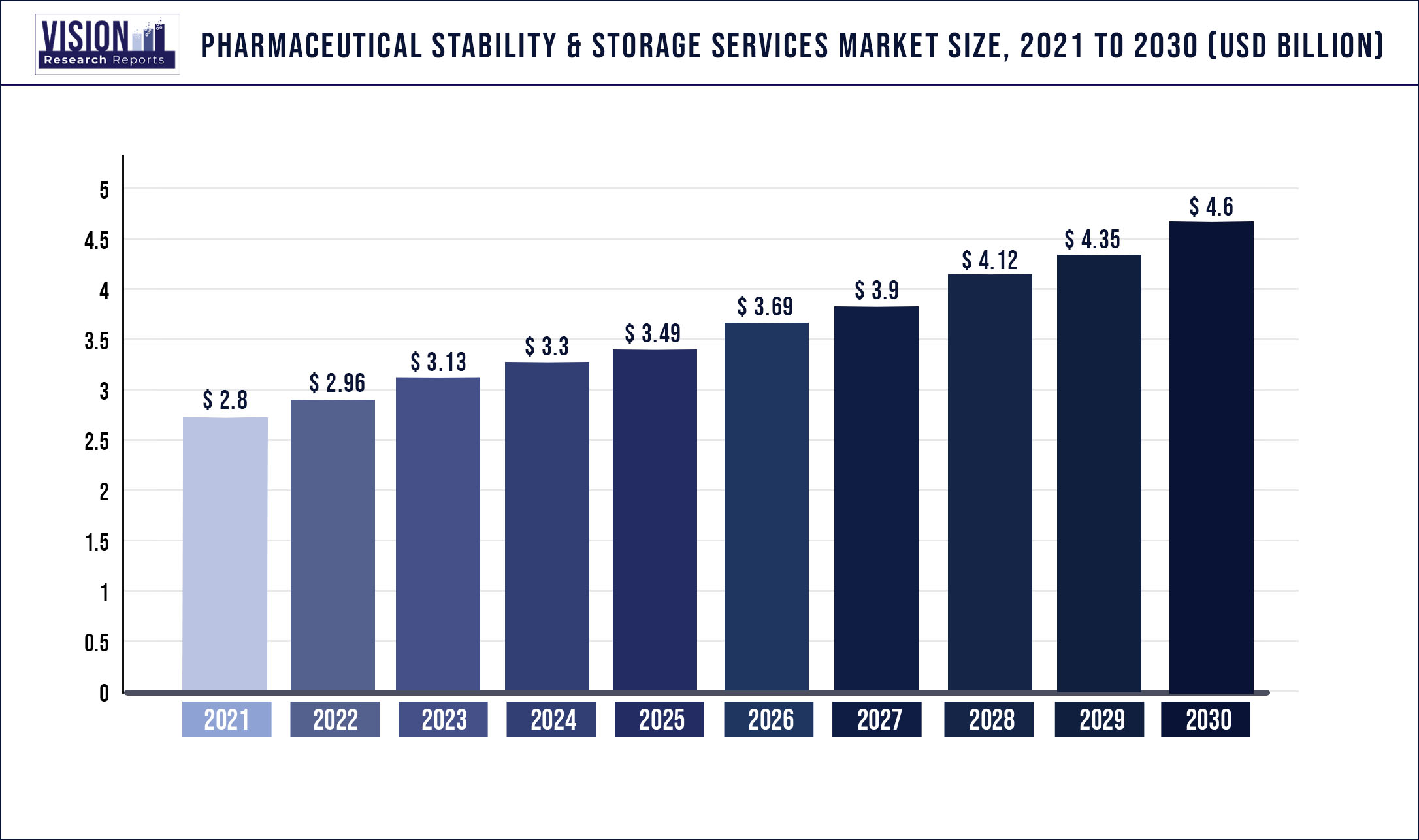
The global pharmaceutical stability & storage services market size was estimated at around USD 2.8 billion in 2021 and it is projected to hit around USD 4.6 billion by 2030, growing at a CAGR of 5.67% from 2022 to 2030.
Stability and storage is a mandatory regulation in various regions. For instance, different regulatory authorities have different data requirements and testing rules for testing stability. Even though FDA and EMA follow the ICH guidelines for stability testing, they still have different microbiological limits for stability tests. This has improved the demand for stability testing outsourcing services and is likely to have a positive impact on the market.
The COVID-19 pandemic had increased the demand for COVID-19 vaccines globally. The growing vaccine drive by the government authorities is likely to drive the demand for stability and storage of commercial COVID-19 vaccines. In recent years pharmaceutical R&D spending has improved significantly. The growing R&D spending is expected to improve the number of drugs in the clinical stage. Stability testing is required for the approval of each phase of a clinical trial. This is further driving the market growth. Moreover, Biosimilar drugs are highly similar copies of biologics and are very cheaper, as compared to biologics.
Biosimilar drugs are widely used in cancer, autoimmune diseases, and other diseases. This is contributing to the demand for biosimilar drugs and thereby is expected to drive the market demand. There has been a rise in several diseases post-COVID-19. For instance, according to a report published by Children’s National Hospital- pediatric research and clinical innovations center, a study was performed on 737 youths who were diagnosed with diabetes, and it found an increased incidence of pediatric Type 1 Diabetes (T1D) by 15.2% and Type 2 Diabetes (T2D) increased by 182% between March 11, 2018, and March 10, 2021. The rise in the disease incidence is expected to improve, drug production, which is likely to drive the market demand.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 2.8 billion |
| Revenue Forecast by 2030 | USD 4.6 billion |
| Growth rate from 2022 to 2030 | CAGR of 5.67% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Services, molecule, mode, region |
| Companies Covered | Catalent Inc.; Almac Group; Charles River Laboratories International, Inc.; Eurofins Scientific SE; Intertek Group plc; Lucideon Limited; Alcami Corporation; Element Materials Technology; BioLife Solutions; Q1 Scientific; Masy BioServices; Reading Scientific Services Ltd.; Roylance Stability Storage Limited; ALS Ltd.'s; Q Laboratories; Auriga Research Private Limited; PD Partners; Precision Stability Storage |
Service Insights
The stability segment dominated the market and accounted for the largest revenue share of 72.8% in 2021. Stability testing is an important parameter that must be analyzed and reported by pharmaceutical companies aiming to gain marketing approvals from the regulatory authorities. The mandatory requirement for stability testing in each phase of the clinical studies is further improving its demand in the market.
The storage segment is expected to register the fastest CAGR of 6.3% during the forecast period. Pharmaceutical drugs are stored in cold and non-cold conditions under environmentally controlled chambers to check whether the quality of the drug changes with time under the environmental conditions of humidity, temperature, and light. The majority of small molecule drugs are required to be stored in non-cold conditions and the high existence of commercially available small molecules is driving the demand for non-cold storage in the market.
Molecule Insights
The small molecule segment accounted for the maximum revenue share of 63.6% in 2021. Over the last three decades, small molecule drug development has advanced dramatically. Small molecules consist of approximately 90% of the total pharmaceutical drugs. It is used in the treatment of fever, migraine, cancer, diabetes, and other common diseases. The use of small-molecule drugs in the treatment of common diseases and disorders is contributing to its demand for stable testing and storage.
The large molecule segment is expected to rise with the fastest CAGR of 6.6%over the forecast period. Large molecules are widely used in the treatment of cancer, infectious diseases, and autoimmune diseases among others. The high burden of these diseases is expected to improve the demand for large molecules and thus is likely to promote the demand for stability and storage of large molecules.
Mode Insights
The in-house segment accounted for the maximum revenue share of 60.2% in the global market in 2021. The majority of pharmaceutical manufacturers choose in-house stability testing, as these tests are required to be performed for all drugs and in all phases of clinical studies. Owning a stability chamber for performing stability studies is considered cheaper in the long run, which is one of the reasons that pharmaceutical companies consider in-house services for stability testing. An in-house team performing stability testing will be more convenient for those pharmaceutical companies that can afford the initial setup cost; these factors are driving the demand for in-house services in the market.
The outsourcing segment is expected to rise with the fastest CAGR of 6.2% during the forecast period. Complications associated with stability testing are driving the demand for outsourcing in the market. Different regulatory authorities have different data requirements and testing rules, which makes it difficult to market products, especially in different markets. For instance, even though EMA and FDA follow ICH guidelines for stability testing, they still have different microbiological requirements for stability tests. Such differences make stability testing complicated, which increases the demand for outsourcing services.
Regional Insights
North America held the largest revenue share 53.7% of the pharmaceutical stability and storage market. This can be attributed to the significant number of pharmaceutical companies in the U.S. and Canada. The presence of major market players providing stability and storage services in this region is expected to contribute significantly to the market growth.
Asia Pacific is expected to grow at the fastest CAGR of 6.8% over the forecast. The region is the fastest growing pharmaceutical market, due to the presence of countries like China, India, and Japan. The initiatives by public organizations to reduce the prices of drugs so as to provide better access to quality medicines have contributed to the market growth. The region is also focused on clinical research. For instance, according to Global data, China had accounted for 26% of global clinical trials trial activity in 2021, and the significant number of clinical trials in these countries are likely to profit the Asia Pacific market.
Key Players
Market Segmentation
Chapter 1. Introduction
1.1.Research Objective
1.2.Scope of the Study
1.3.Definition
Chapter 2. Research Methodology
2.1.Research Approach
2.2.Data Sources
2.3.Assumptions & Limitations
Chapter 3. Executive Summary
3.1.Market Snapshot
Chapter 4. Market Variables and Scope
4.1.Introduction
4.2.Market Classification and Scope
4.3.Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Mode Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Pharmaceutical Stability & Storage Services Market
5.1.COVID-19 Landscape: Pharmaceutical Stability & Storage Services Industry Impact
5.2.COVID 19 - Impact Assessment for the Industry
5.3.COVID 19 Impact: Global Major Government Policy
5.4.Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1.Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2.Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Pharmaceutical Stability & Storage Services Market, By Services
8.1.Pharmaceutical Stability & Storage Services Market, by Services Type, 2022-2030
8.1.1. Stability
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Storage
8.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Pharmaceutical Stability & Storage Services Market, By Molecule
9.1.Pharmaceutical Stability & Storage Services Market, by Molecule, 2022-2030
9.1.1. Small Molecule
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Large Molecule
9.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 10.Global Pharmaceutical Stability & Storage Services Market, By Mode
10.1.Pharmaceutical Stability & Storage Services Market, by Mode, 2022-2030
10.1.1.In-house
10.1.1.1.Market Revenue and Forecast (2017-2030)
10.1.2.Outsourcing
10.1.2.1.Market Revenue and Forecast (2017-2030)
Chapter 11.Global Pharmaceutical Stability & Storage Services Market, Regional Estimates and Trend Forecast
11.1.North America
11.1.1.Market Revenue and Forecast, by Services (2017-2030)
11.1.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.1.3.Market Revenue and Forecast, by Mode (2017-2030)
11.1.4.U.S.
11.1.4.1.Market Revenue and Forecast, by Services (2017-2030)
11.1.4.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.1.4.3.Market Revenue and Forecast, by Mode (2017-2030)
11.1.5.Rest of North America
11.1.5.1.Market Revenue and Forecast, by Services (2017-2030)
11.1.5.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.1.5.3.Market Revenue and Forecast, by Mode (2017-2030)
11.2.Europe
11.2.1.Market Revenue and Forecast, by Services (2017-2030)
11.2.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.2.3.Market Revenue and Forecast, by Mode (2017-2030)
11.2.4.UK
11.2.4.1.Market Revenue and Forecast, by Services (2017-2030)
11.2.4.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.2.4.3.Market Revenue and Forecast, by Mode (2017-2030)
11.2.5.Germany
11.2.5.1.Market Revenue and Forecast, by Services (2017-2030)
11.2.5.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.2.5.3.Market Revenue and Forecast, by Mode (2017-2030)
11.2.6.France
11.2.6.1.Market Revenue and Forecast, by Services (2017-2030)
11.2.6.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.2.6.3.Market Revenue and Forecast, by Mode (2017-2030)
11.2.7.Rest of Europe
11.2.7.1.Market Revenue and Forecast, by Services (2017-2030)
11.2.7.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.2.7.3.Market Revenue and Forecast, by Mode (2017-2030)
11.3.APAC
11.3.1.Market Revenue and Forecast, by Services (2017-2030)
11.3.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.3.3.Market Revenue and Forecast, by Mode (2017-2030)
11.3.4.India
11.3.4.1.Market Revenue and Forecast, by Services (2017-2030)
11.3.4.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.3.4.3.Market Revenue and Forecast, by Mode (2017-2030)
11.3.5.China
11.3.5.1.Market Revenue and Forecast, by Services (2017-2030)
11.3.5.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.3.5.3.Market Revenue and Forecast, by Mode (2017-2030)
11.3.6.Japan
11.3.6.1.Market Revenue and Forecast, by Services (2017-2030)
11.3.6.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.3.6.3.Market Revenue and Forecast, by Mode (2017-2030)
11.3.7.Rest of APAC
11.3.7.1.Market Revenue and Forecast, by Services (2017-2030)
11.3.7.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.3.7.3.Market Revenue and Forecast, by Mode (2017-2030)
11.4.MEA
11.4.1.Market Revenue and Forecast, by Services (2017-2030)
11.4.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.4.3.Market Revenue and Forecast, by Mode (2017-2030)
11.4.4.GCC
11.4.4.1.Market Revenue and Forecast, by Services (2017-2030)
11.4.4.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.4.4.3.Market Revenue and Forecast, by Mode (2017-2030)
11.4.5.North Africa
11.4.5.1.Market Revenue and Forecast, by Services (2017-2030)
11.4.5.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.4.5.3.Market Revenue and Forecast, by Mode (2017-2030)
11.4.6.South Africa
11.4.6.1.Market Revenue and Forecast, by Services (2017-2030)
11.4.6.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.4.6.3.Market Revenue and Forecast, by Mode (2017-2030)
11.4.7.Rest of MEA
11.4.7.1.Market Revenue and Forecast, by Services (2017-2030)
11.4.7.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.4.7.3.Market Revenue and Forecast, by Mode (2017-2030)
11.5.Latin America
11.5.1.Market Revenue and Forecast, by Services (2017-2030)
11.5.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.5.3.Market Revenue and Forecast, by Mode (2017-2030)
11.5.4.Brazil
11.5.4.1.Market Revenue and Forecast, by Services (2017-2030)
11.5.4.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.5.4.3.Market Revenue and Forecast, by Mode (2017-2030)
11.5.5.Rest of LATAM
11.5.5.1.Market Revenue and Forecast, by Services (2017-2030)
11.5.5.2.Market Revenue and Forecast, by Molecule (2017-2030)
11.5.5.3.Market Revenue and Forecast, by Mode (2017-2030)
Chapter 12.Company Profiles
12.1.Catalent Inc.
12.1.1.Company Overview
12.1.2.Product Offerings
12.1.3.Financial Performance
12.1.4.Recent Initiatives
12.2.Almac Group
12.2.1.Company Overview
12.2.2.Product Offerings
12.2.3.Financial Performance
12.2.4.Recent Initiatives
12.3.Charles River Laboratories International, Inc.
12.3.1.Company Overview
12.3.2.Product Offerings
12.3.3.Financial Performance
12.3.4.Recent Initiatives
12.4.Eurofins Scientific SE
12.4.1.Company Overview
12.4.2.Product Offerings
12.4.3.Financial Performance
12.4.4.Recent Initiatives
12.5.Intertek Group plc
12.5.1.Company Overview
12.5.2.Product Offerings
12.5.3.Financial Performance
12.5.4.Recent Initiatives
12.6.Lucideon Limited
12.6.1.Company Overview
12.6.2.Product Offerings
12.6.3.Financial Performance
12.6.4.Recent Initiatives
12.7.Alcami Corporation
12.7.1.Company Overview
12.7.2.Product Offerings
12.7.3.Financial Performance
12.7.4.Recent Initiatives
12.8.Element Materials Technology
12.8.1.Company Overview
12.8.2.Product Offerings
12.8.3.Financial Performance
12.8.4.Recent Initiatives
12.9.BioLife Solutions
12.9.1.Company Overview
12.9.2.Product Offerings
12.9.3.Financial Performance
12.9.4.Recent Initiatives
12.10.Q1 Scientific
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
12.11.Masy BioServices
12.11.1. Company Overview
12.11.2. Product Offerings
12.11.3. Financial Performance
12.11.4. Recent Initiatives
12.12.Reading Scientific Services Ltd.
12.12.1. Company Overview
12.12.2. Product Offerings
12.12.3. Financial Performance
12.12.4. Recent Initiatives
12.13.Roylance Stability Storage Limited
12.13.1. Company Overview
12.13.2. Product Offerings
12.13.3. Financial Performance
12.13.4. Recent Initiatives
12.14.ALS Ltd.'s
12.14.1. Company Overview
12.14.2. Product Offerings
12.14.3. Financial Performance
12.14.4. Recent Initiatives
12.15.Q Laboratories
12.15.1. Company Overview
12.15.2. Product Offerings
12.15.3. Financial Performance
12.15.4. Recent Initiatives
12.16.Auriga Research Private Limited
12.16.1. Company Overview
12.16.2. Product Offerings
12.16.3. Financial Performance
12.16.4. Recent Initiatives
12.17.PD Partners
12.17.1. Company Overview
12.17.2. Product Offerings
12.17.3. Financial Performance
12.17.4. Recent Initiatives
12.18.Precision Stability Storage
12.18.1. Company Overview
12.18.2. Product Offerings
12.18.3. Financial Performance
12.18.4. Recent Initiatives
Chapter 13.Research Methodology
13.1.Primary Research
13.2.Secondary Research
13.3.Assumptions
Chapter 14.Appendix
14.1.About Us
14.2.Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others