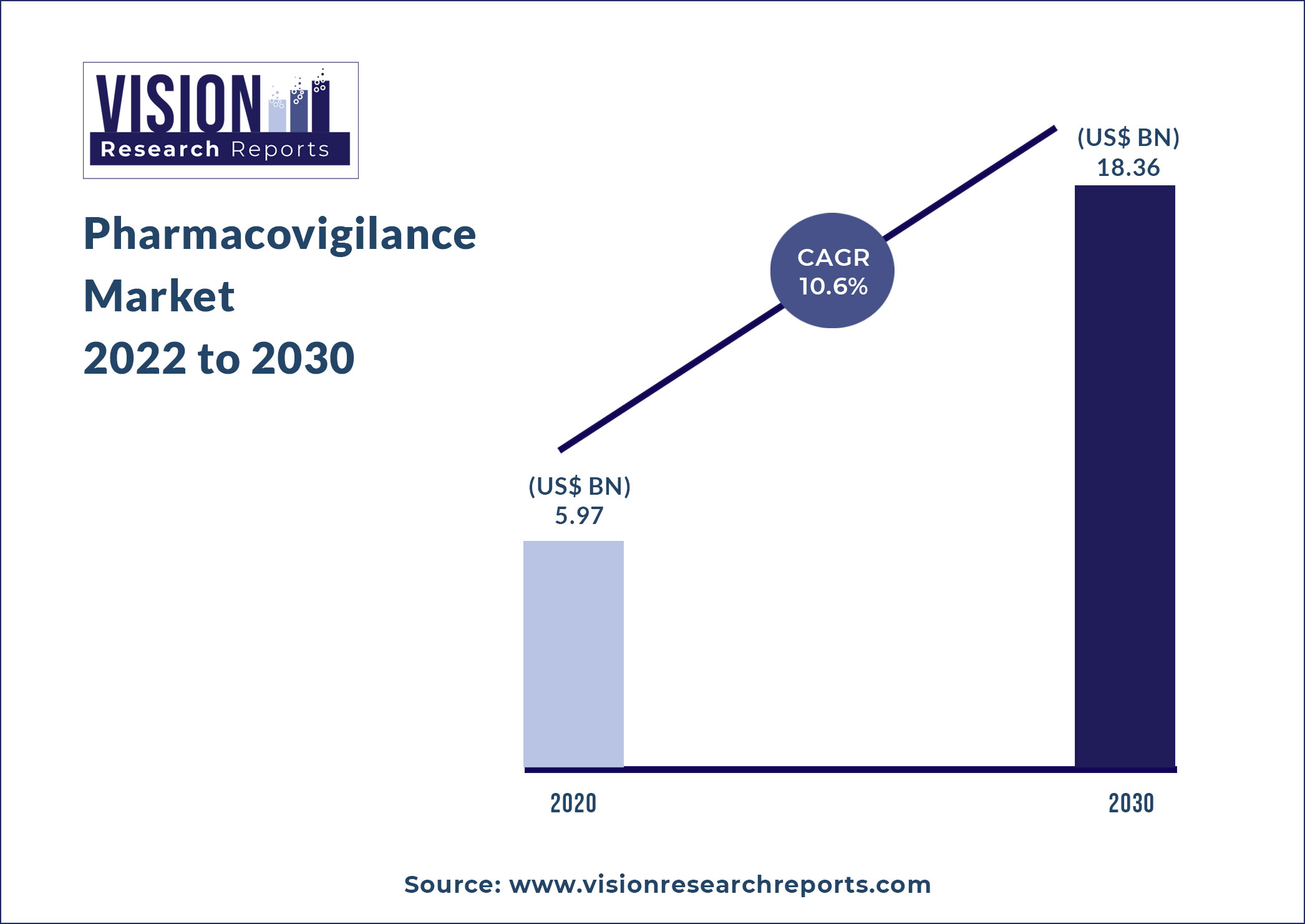
The global pharmacovigilance market size was valued at USD 5.97 billion in 2020, and is predicted to be worth around USD 18.36 billion by 2030, registering a CAGR of 10.6% during the forecast period 2022 to 2030.
Growth Factors
Increasing incidence of Adverse Drug Reactions (ADRs) is the key growth driver. ADR imposes a substantial burden on healthcare systems and is one of the prominent causes of morbidity in developed countries. According to the National Center for Biotechnology Information (NCBI), approximately 5% of total hospitalizations in Europe each year are due to ADR. Pharmacovigilance services play an integral role in this clinical trial phase by assisting manufacturers in identifying adverse effects associated with the drug.
An increase in the prevalence of chronic diseases such as oncological diseases, diabetes, and cardiovascular and respiratory disorders has led to an increase in drug consumption worldwide. Therefore, the demand for new drug development via extensive clinical trials has increased. Pharmacovigilance (PV) is the inevitable part of drug discovery and development procedures.
Report Coverage
| Report Scope | Details |
| Market Size | US$ 18.36 billion by 2030 |
| Growth Rate | CAGR of 10.6% From 2022 to 2030 |
| Largest Market | North America |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Service Provider, Product life cycle, Type, Process Flow, Therapeutic area, End-use |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Mentioned | Accenture; Linical Accelovance; Cognizant; Laboratory Corporation of America Holdings; IBM Corporation; ArisGlobal; ICON plc.; Capgemini; ITClinical; FMD K&L; IQVIA; TAKE Solutions Ltd.; PAREXEL International Corporation; BioClinica Inc.; Wipro Ltd.; United BioSource Corporation |
By Service Provider Analysis
Contract outsourcing held the largest share of over 55.0% in 2021 and is expected to witness the fastest growth in the forthcoming years. The growth can be attributed to the benefits associated with outsourcing such as risk mitigation, resource flexibility, reduction of upfront investments, and lower fixed cost.
The dynamic growth of the contract outsourcing segment can also be attributed to the rapidly emerging CROs providing end-to-end clinical trial solutions, especially in the emerging economies of Asia Pacific, such as India, China, and Japan, enabling resources sharing, cost efficiency, resource flexibility, and the expansion of operative capabilities. Contract outsourcing also helps reduce the complexity of clinical trials, allows faster approval of trials, and helps effective utilization of internal resources.
By Product Life Cycle Analysis
The phase IV (post-marketing) segment led the overall market with a revenue share of over 75.0% in 2021. These solutions act as an additional safety measure for the drugs undergoing clinical trials.
The phase III segment is expected to witness lucrative growth over the forecast period. Phase III trials are done to determine and establish the efficacy of drugs. These trials also provide additional information regarding possible drug interactions, drug safety, and effectiveness before the commercialization of the drug.
By Type Analysis
Spontaneous reporting held the largest share of over 30.0% in 2021 owing to wide usage in the detection of new, serious, and rare ADRs and serves as an efficient and inexpensive method.
Cohort Event Monitoring (CEM) emerged as the second-largest segment in 2021 owing to the increasing application in the detection of a wide range of adverse clinical events. Conjugation of CEM with statistical tools and data mining systems such as longitudinal health records are responsible for the growing popularity of this type.
Targeted spontaneous reporting is projected to be the fastest-growing segment over the forecast period owing to the rising government initiatives to incorporate reporting methodologies other than spontaneous reporting by the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP).
Electronic Health Record (EHR) mining is increasingly used to identify risk factors for patients after discharge from hospitals. Electronic health records are imperative sources of medical information about clinical events in hospitals and research organizations.
By Therapeutic Area Analysis
The oncology segment held the largest revenue share of over 25.0% in 2021. Monitoring the safety of cancer drugs is very important due to the associated side effects, which is propelling the demand for pharmacovigilance services.
Pharmacovigilance helps in the early detection and spontaneous reporting of adverse drug reactions. Moreover, recent advancements in cancer treatments, such as targeted therapy, have some serious adverse effects and can compromise a patient’s quality of life.
By Process Flow Analysis
Signal detection dominated the market with a revenue share of over 35.0% in 2021. Spontaneous Reporting Systems (SRSs) use the dominant source of signals through which the suspected cases get voluntarily reported by the healthcare professionals to the other regulatory bodies.
Artificial Intelligence (AI) and big data are being used by companies for better assessment of signals. The case data management segment is expected to exhibit the fastest growth rate over the forecast period.
By End-use Analysis
Pharmaceuticals held the largest revenue share of over 40.0% in terms of revenue. Outsourcing the pharmacovigilance process is practiced by pharma companies to avoid high upfront investments and fixed overhead costs, increase resource flexibility, and secure additional capacity.
The biotechnology segment is anticipated to witness lucrative growth in the forthcoming years owing to increasing new product development activities in this sector. In recent years, drugs are being developed and consumed at increasingly high rates.
By Regional Analysis
North America held the largest revenue share of over 30.0% in 2021 owing to the presence of key pharmaceutical and medical devices players, contributing to the overall revenue in this region.
Asia Pacific is expected to register the fastest CAGR of 12.1% during the forecast period owing to the availability of various outsourcing organizations. Consequentially, there is improved productivity, cost efficiency, and resource sharing that is anticipated to propel the regional demand for pharmacovigilance in the forthcoming years.
Key Players
Accenture
Linical Accelovance
Cognizant
Laboratory Corporation of America Holdings
IBM Corporation
ArisGlobal
ICON plc.
Capgemini
ITClinical
FMD K&L
IQVIA
TAKE Solutions Ltd.
PAREXEL International Corporation
BioClinica Inc.
Wipro Ltd.
United BioSource Corporation
Market Segmentation
By Service Provider
In-house
Contract Outsourcing
By Product Life Cycle
Pre-clinical
Phase I
Phase II
Phase III
Phase IV
By Type
Spontaneous Reporting
Intensified ADR Reporting
Targeted Spontaneous Reporting
Cohort Event Monitoring
EHR Mining
By Process Flow
Case Data Management
Case Logging
Case Data Analysis
Medical Reviewing & Reporting
Signal Detection
Adverse Event Logging
Adverse Event Analysis
Adverse Event Review & Reporting
Risk Management System
Risk Evaluation System
Risk Mitigation System
By Therapeutic Area
Oncology
Neurology
Cardiology
Respiratory Systems
Others
By End-use
Pharmaceuticals
Biotechnology Companies
Medical Device Manufacturers
Others
Regional
North America
U.S.
Canada
Europe
U.K.
Germany
France
Italy
Spain
Russia
Asia Pacific
Japan
China
India
Latin America
Brazil
Mexico
Middle East and Africa
South Africa
Kingdom of Saudi Arabia
The pharmacovigilance market research report covers definition, classification, product classification, product application, development trend, product technology, competitive landscape, industrial chain structure, industry overview, national policy and planning analysis of the industry, the latest dynamic analysis, etc., and also includes major. The study includes drivers and restraints of the global market. It covers the impact of these drivers and restraints on the demand during the forecast period. The report also highlights opportunities in the market at the global level.
The report provides size (in terms of volume and value) of pharmacovigilance market for the base year 2021 and the forecast between 2022 and 2030. Market numbers have been estimated based on form and application. Market size and forecast for each application segment have been provided for the global and regional market.
This report focuses on the global pharmacovigilance market status, future forecast, growth opportunity, key market and key players. The study objectives are to present the pharmacovigilance market development in United States, Europe and China.
It is pertinent to consider that in a volatile global economy, we haven’t just conducted pharmacovigilance market forecasts in terms of CAGR, but also studied the market based on key parameters, including Year-on-Year (Y-o-Y) growth, to comprehend the certainty of the market and to find and present the lucrative opportunities in market.
In terms of production side, this report researches the pharmacovigilance capacity, production, value, ex-factory price, growth rate, market share for major manufacturers, regions (or countries) and type.
In terms of consumption side, this report focuses on the consumption of pharmacovigilance by regions (countries) and application.
Buyers of the report will have access to verified market figures, including global market size in terms of revenue and volume. As part of production analysis, the authors of the report have provided reliable estimations and calculations for global revenue and volume by Type segment of the global pharmacovigilance market. These figures have been provided in terms of both revenue and volume for the period 2019 to 2030. Additionally, the report provides accurate figures for production by region in terms of revenue as well as volume for the same period. The report also includes production capacity statistics for the same period.
With regard to production bases and technologies, the research in this report covers the production time, base distribution, technical parameters, research and development trends, technology sources, and sources of raw materials of major pharmacovigilance market companies.
Regarding the analysis of the industry chain, the research of this report covers the raw materials and equipment of pharmacovigilance market upstream, downstream customers, marketing channels, industry development trends and investment strategy recommendations. The more specific analysis also includes the main application areas of market and consumption, major regions and Consumption, major Chinese producers, distributors, raw material suppliers, equipment providers and their contact information, industry chain relationship analysis.
The research in this report also includes product parameters, production process, cost structure, and data information classified by region, technology and application. Finally, the paper model new project SWOT analysis and investment feasibility study of the case model.
Overall, this is an in-depth research report specifically for the pharmacovigilance industry. The research center uses an objective and fair way to conduct an in-depth analysis of the development trend of the industry, providing support and evidence for customer competition analysis, development planning, and investment decision-making. In the course of operation, the project has received support and assistance from technicians and marketing personnel in various links of the industry chain.
pharmacovigilance market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies’ focus related to pharmacovigilance market.
Prominent players in the market are predicted to face tough competition from the new entrants. However, some of the key players are targeting to acquire the startup companies in order to maintain their dominance in the global market. For a detailed analysis of key companies, their strengths, weaknesses, threats, and opportunities are measured in the report by using industry-standard tools such as the SWOT analysis. Regional coverage of key companies is covered in the report to measure their dominance. Key manufacturers of pharmacovigilance market are focusing on introducing new products to meet the needs of the patrons. The feasibility of new products is also measured by using industry-standard tools.
Key companies are increasing their investments in research and development activities for the discovery of new products. There has also been a rise in the government funding for the introduction of new pharmacovigilance market. These factors have benefited the growth of the global market for pharmacovigilance. Going forward, key companies are predicted to benefit from the new product launches and the adoption of technological advancements. Technical advancements have benefited many industries and the global industry is not an exception.
New product launches and the expansion of already existing business are predicted to benefit the key players in maintaining their dominance in the global market for pharmacovigilance. The global market is segmented on the basis of region, application, end-users and product type. Based on region, the market is divided into North America, Europe, Asia-Pacific, Latin America and Middle East and Africa (MEA).
In this study, the years considered to estimate the market size of pharmacovigilance are as follows:
Reasons to Purchase this Report:
- Market segmentation analysis including qualitative and quantitative research incorporating the impact of economic and policy aspects
- Regional and country level analysis integrating the demand and supply forces that are influencing the growth of the market.
- Market value USD Million and volume Units Million data for each segment and sub-segment
- Competitive landscape involving the market share of major players, along with the new projects and strategies adopted by players in the past five years
- Comprehensive company profiles covering the product offerings, key financial information, recent developments, SWOT analysis, and strategies employed by the major market players
Research Methodology:
In-depth interviews and discussions were conducted with several key market participants and opinion leaders to compile the research report.
This research study involved the extensive usage of both primary and secondary data sources. The research process involved the study of various factors affecting the industry, including the government policy, market environment, competitive landscape, historical data, present trends in the market, technological innovation, upcoming technologies and the technical progress in related industry, and market risks, opportunities, market barriers and challenges. The following illustrative figure shows the market research methodology applied in this report.
The study objectives of this report are:
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Pharmacovigilance Market, By Service Provider
7.1. Pharmacovigilance Market, by Service Provider, 2021-2030
7.1.1. In-house
7.1.1.1. Market Revenue and Forecast (2017-2030)
7.1.2. Contract Outsourcing
7.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 8. Global Pharmacovigilance Market, By Product Life Cycle
8.1. Pharmacovigilance Market, by Product Life Cycle, 2021-2030
8.1.1. Pre-clinical
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Phase I
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Phase II
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Phase III
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Phase IV
8.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Pharmacovigilance Market, By Type
9.1. Pharmacovigilance Market, by Type, 2021-2030
9.1.1. Spontaneous Reporting
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Intensified ADR Reporting
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Targeted Spontaneous Reporting
9.1.3.1. Market Revenue and Forecast (2017-2030)
9.1.4. Cohort Event Monitoring
9.1.4.1. Market Revenue and Forecast (2017-2030)
9.1.5. EHR Mining
9.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Pharmacovigilance Market, By Process Flow
10.1. Pharmacovigilance Market, by Process Flow, 2021-2030
10.1.1. Case Data Management
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Signal Detection
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Risk Management System
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Pharmacovigilance Market, By Therapeutic Area
11.1. Pharmacovigilance Market, by Therapeutic Area, 2021-2030
11.1.1. Oncology
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Neurology
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Cardiology
11.1.3.1. Market Revenue and Forecast (2017-2030)
11.1.4. Respiratory Systems
11.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global Pharmacovigilance Market, By End-use
12.1. Pharmacovigilance Market, by End-use, 2021-2030
12.1.1. Pharmaceuticals
12.1.1.1. Market Revenue and Forecast (2017-2030)
12.1.2. Biotechnology Companies
12.1.2.1. Market Revenue and Forecast (2017-2030)
12.1.3. Medical Device Manufacturers
12.1.3.1. Market Revenue and Forecast (2017-2030)
12.1.4. Others
12.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 13. Global Pharmacovigilance Market, Regional Estimates and Trend Forecast
13.1. North America
13.1.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.1.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.1.3. Market Revenue and Forecast, by Type (2017-2030)
13.1.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.1.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.1.6. Market Revenue and Forecast, by End-use (2017-2030)
13.1.7. U.S.
13.1.7.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.1.7.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.1.7.3. Market Revenue and Forecast, by Type (2017-2030)
13.1.7.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.1.7.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.1.7.6. Market Revenue and Forecast, by End-use (2017-2030)
13.1.8. Rest of North America
13.1.8.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.1.8.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.1.8.3. Market Revenue and Forecast, by Type (2017-2030)
13.1.8.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.1.8.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.1.8.6. Market Revenue and Forecast, by End-use (2017-2030)
13.2. Europe
13.2.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.2.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.2.3. Market Revenue and Forecast, by Type (2017-2030)
13.2.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.2.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.2.6. Market Revenue and Forecast, by End-use (2017-2030)
13.2.7. UK
13.2.7.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.2.7.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.2.7.3. Market Revenue and Forecast, by Type (2017-2030)
13.2.7.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.2.7.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.2.7.6. Market Revenue and Forecast, by End-use (2017-2030)
13.2.8. Germany
13.2.8.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.2.8.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.2.8.3. Market Revenue and Forecast, by Type (2017-2030)
13.2.8.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.2.8.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.2.8.6. Market Revenue and Forecast, by End-use (2017-2030)
13.2.9. France
13.2.9.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.2.9.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.2.9.3. Market Revenue and Forecast, by Type (2017-2030)
13.2.9.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.2.9.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.2.9.6. Market Revenue and Forecast, by End-use (2017-2030)
13.2.10. Rest of Europe
13.2.10.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.2.10.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.2.10.3. Market Revenue and Forecast, by Type (2017-2030)
13.2.10.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.2.10.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.2.10.6. Market Revenue and Forecast, by End-use (2017-2030)
13.3. APAC
13.3.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.3.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.3.3. Market Revenue and Forecast, by Type (2017-2030)
13.3.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.3.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.3.6. Market Revenue and Forecast, by End-use (2017-2030)
13.3.7. India
13.3.7.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.3.7.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.3.7.3. Market Revenue and Forecast, by Type (2017-2030)
13.3.7.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.3.7.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.3.7.6. Market Revenue and Forecast, by End-use (2017-2030)
13.3.8. China
13.3.8.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.3.8.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.3.8.3. Market Revenue and Forecast, by Type (2017-2030)
13.3.8.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.3.8.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.3.8.6. Market Revenue and Forecast, by End-use (2017-2030)
13.3.9. Japan
13.3.9.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.3.9.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.3.9.3. Market Revenue and Forecast, by Type (2017-2030)
13.3.9.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.3.9.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.3.9.6. Market Revenue and Forecast, by End-use (2017-2030)
13.3.10. Rest of APAC
13.3.10.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.3.10.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.3.10.3. Market Revenue and Forecast, by Type (2017-2030)
13.3.10.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.3.10.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.3.10.6. Market Revenue and Forecast, by End-use (2017-2030)
13.4. MEA
13.4.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.4.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.4.3. Market Revenue and Forecast, by Type (2017-2030)
13.4.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.4.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.4.6. Market Revenue and Forecast, by End-use (2017-2030)
13.4.7. GCC
13.4.7.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.4.7.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.4.7.3. Market Revenue and Forecast, by Type (2017-2030)
13.4.7.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.4.7.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.4.7.6. Market Revenue and Forecast, by End-use (2017-2030)
13.4.8. North Africa
13.4.8.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.4.8.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.4.8.3. Market Revenue and Forecast, by Type (2017-2030)
13.4.8.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.4.8.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.4.8.6. Market Revenue and Forecast, by End-use (2017-2030)
13.4.9. South Africa
13.4.9.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.4.9.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.4.9.3. Market Revenue and Forecast, by Type (2017-2030)
13.4.9.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.4.9.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.4.9.6. Market Revenue and Forecast, by End-use (2017-2030)
13.4.10. Rest of MEA
13.4.10.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.4.10.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.4.10.3. Market Revenue and Forecast, by Type (2017-2030)
13.4.10.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.4.10.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.4.10.6. Market Revenue and Forecast, by End-use (2017-2030)
13.5. Latin America
13.5.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.5.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.5.3. Market Revenue and Forecast, by Type (2017-2030)
13.5.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.5.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.5.6. Market Revenue and Forecast, by End-use (2017-2030)
13.5.7. Brazil
13.5.7.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.5.7.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.5.7.3. Market Revenue and Forecast, by Type (2017-2030)
13.5.7.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.5.7.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.5.7.6. Market Revenue and Forecast, by End-use (2017-2030)
13.5.8. Rest of LATAM
13.5.8.1. Market Revenue and Forecast, by Service Provider (2017-2030)
13.5.8.2. Market Revenue and Forecast, by Product Life Cycle (2017-2030)
13.5.8.3. Market Revenue and Forecast, by Type (2017-2030)
13.5.8.4. Market Revenue and Forecast, by Process Flow (2017-2030)
13.5.8.5. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
13.5.8.6. Market Revenue and Forecast, by End-use (2017-2030)
Chapter 14. Company Profiles
14.1. Accenture
14.1.1. Company Overview
14.1.2. Product Offerings
14.1.3. Financial Performance
14.1.4. Recent Initiatives
14.2. Linical Accelovance
14.2.1. Company Overview
14.2.2. Product Offerings
14.2.3. Financial Performance
14.2.4. Recent Initiatives
14.3. Cognizant
14.3.1. Company Overview
14.3.2. Product Offerings
14.3.3. Financial Performance
14.3.4. Recent Initiatives
14.4. Laboratory Corporation of America Holdings
14.4.1. Company Overview
14.4.2. Product Offerings
14.4.3. Financial Performance
14.4.4. Recent Initiatives
14.5. IBM Corporation
14.5.1. Company Overview
14.5.2. Product Offerings
14.5.3. Financial Performance
14.5.4. Recent Initiatives
14.6. ArisGlobal
14.6.1. Company Overview
14.6.2. Product Offerings
14.6.3. Financial Performance
14.6.4. Recent Initiatives
14.7. ICON plc.
14.7.1. Company Overview
14.7.2. Product Offerings
14.7.3. Financial Performance
14.7.4. Recent Initiatives
14.8. Capgemini
14.8.1. Company Overview
14.8.2. Product Offerings
14.8.3. Financial Performance
14.8.4. Recent Initiatives
14.9. ITClinical
14.9.1. Company Overview
14.9.2. Product Offerings
14.9.3. Financial Performance
14.9.4. Recent Initiatives
14.10. FMD K&L
14.10.1. Company Overview
14.10.2. Product Offerings
14.10.3. Financial Performance
14.10.4. Recent Initiatives
Chapter 15. Research Methodology
15.1. Primary Research
15.2. Secondary Research
15.3. Assumptions
Chapter 16. Appendix
16.1. About Us
Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others