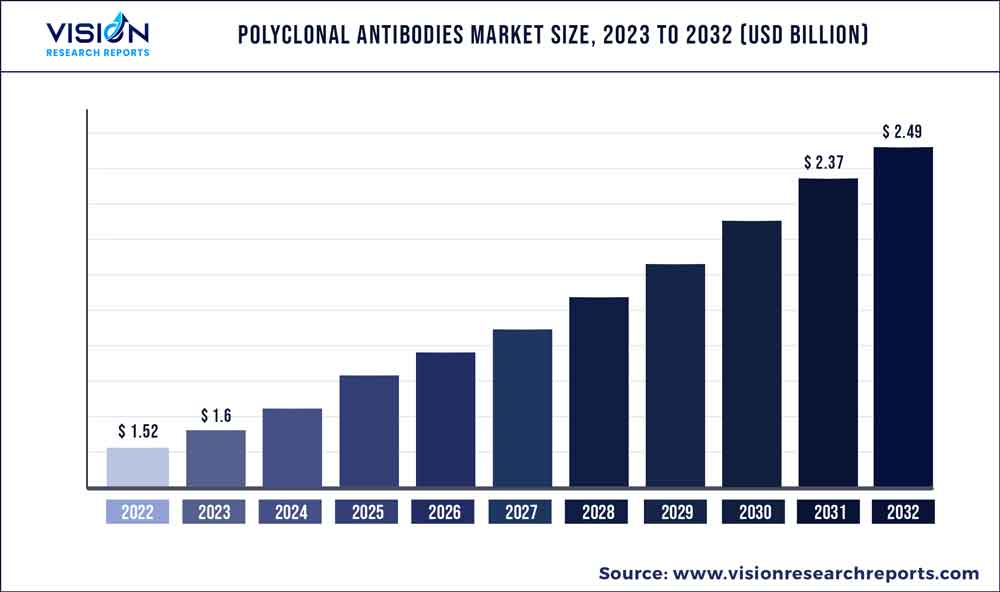
The global polyclonal antibodies market was estimated at USD 1.52 billion in 2022 and it is expected to surpass around USD 2.49 billion by 2032, poised to grow at a CAGR of 5.04% from 2023 to 2032. The polyclonal antibodies market in the United States was accounted for USD 581 million in 2022.
Key Pointers
Report Scope of the Polyclonal Antibodies Market
| Report Coverage | Details |
| Revenue Share of North America in 2022 | 39% |
| CAGR of Asia Pacific from 2023 to 2032 | 9.69% |
| Revenue Forecast by 2032 | USD 2.49 billion |
| Growth Rate from 2023 to 2032 | CAGR of 5.04% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Companies Covered | Thermo Fisher Scientific Inc.; Merck KgaA; Abcam plc.; ProteoGenix; Proteintech Group, Inc.; Bio-Rad Laboratories Inc.; BPS Bioscience, Inc.; R&D Systems, Inc.; Agilent Technologies, Inc.; Atlas Antibodies; CUSABIO TECHNOLOGY LLC; ROCKLAND IMMUNOCHEMICALS, INC. |
This is mainly attributed to the growing prevalence of infectious and chronic diseases such as cancer across the globe, increasing technological advancements in antibody-based drugs, and the rising R&D activities in the biopharmaceutical industry. Moreover, as compared to monoclonal antibodies, the manufacturing cost of polyclonal antibodies is less. Polyclonal antibodies (pAbs) are widely used in qualitative and quantitative biological analysis and various diagnostic testing. The COVID-19 pandemic significantly impacted the polyclonal antibody market. Various firms are working towards establishing pAbs-based in-house products that are used for treating individuals suffering from COVID-19. The rising acceptance of standard laboratory tests, such as western blot analysis, microarray assays, cell imaging, and immunohistochemical, is projected to propel polyclonal antibody market growth. For instance, GenScript, a U.S.-based biotechnology company, offers tailored pAbs that are appropriate for several assays type, such as CHiP, sandwich ELISA, Immunoprecipitation, Western Blot, IHC, IF, and Flow Cytometry. Additionally, according to an article published in April 2021, a human-derived antibody, SAB-185 developed by SAB Biotherapeutics, Inc., has been tested in a Phase 2/3 trial that is enrolled in non-hospitalized individuals with mild or moderate COVID-19 infections. Hence, these initiatives showcased the rise of demand for the pAbs during the pandemic period.
According to a report published by WHO in February 2022, approximately 10 million deaths were reported in the year 2020, among which, the most common cancers include lung, breast, rectum & colon, and prostate cancer. Furthermore, as per the article released by the American Cancer Society in January 2023, nearly 59,610 fresh leukemia cases (of all kinds) and 23,710 deaths caused due to leukemia (of all kinds) in the U.S. Polyclonal antibody treatment is widely used to treat various cancers including lymphoma, leukemia, solid tumor, and many more. For instance, in August 2021, the Cancer Prevention & Research Institute of Texas was funded with USD 250,000, for Antibody like Therapeutics which would Target Polyclonal T Cells to CMV-Positive Glioblastomas. Thus, the increasing prevalence of cancer is expected to create huge opportunities to propel the upsurge of the market.
According to an article published by the National Library of Medicine in May 2018, the rabbit model was adopted initially to study polyclonal antibody reactions and supported the immunogenicity associated with DNA immunization as an innovative immunization technique. Additionally, the rabbit model has been used significantly in the development of the HIV vaccine. Rabbits have a number of benefits over other small animals, such as mouse or rat, including, ease in inducing high-titer, high-affinity epitope-specific Abs that respond to nearly all kinds of antigens and minimal non-specific responses.
Additionally, government organizations around the world are increasingly recognizing the potential of the pAbs, and are taking initiatives to support its growth. These initiatives are aimed at promoting research and development, improving healthcare infrastructure, and providing funding for the development of new and innovative products. In September 2021, Regeneron Pharmaceuticals, Inc. announced their collaboration with the U.S. government, i.e., the Department of Defense (DOD) and the Department of Health and Human Services (HHS) for buying doses of REGEN-COV (casirivimab and imdevimab) antibody cocktail that is used in treating COVID infected patients in various hospitals.
Product Insights
In 2022, the secondary antibodies segment dominated the market with a market share of 57%. Secondary Abs bind to primary Abs that are raised against the target antigen and amplify the signal through various detection methods including ELISA, Western blotting, and Immunohistochemistry. In addition, secondary Abs are typically produced by immunizing host animals, such as rabbits, goats, or mice along with the immunoglobulin (Ig) of the same species as the primary antibody. Thus, this leads to the production of polyclonal secondary Abs that can recognize multiple epitopes on primary Abs, henceforth increasing the sensitivity and specificity of the assay.
The primary antibodies segment is expected to grow at the fastest CAGR of 6.48% during the forecast period. The increasing adoption of primary antibodies based (pAb-based) assays in clinical diagnostics, and rising demand for high-quality & diverse primary Abs are the factors contributing to the growth of the segment. Primary Abs are essential reagents for various clinical assays, including Western Blotting, ELISA, and Immunohistochemistry for the identification of disease biomarkers. Thus, as the demand for personalized medicine and biomarker-based diagnostics continues to grow, the primary antibody segment in the pAbs market is expected to grow further.
Application Insights
The diagnostics segment dominates the market for polyclonal antibodies with a share of 60% in 2022 and the same is anticipated to have the highest CAGR rate of 5.63% from 2023-2032. pAbs are considered to be an ideal reagent in hemagglutination reactions and diagnostics assay due to their capability to identify the epitopes of targeted molecules. Moreover, pAbs are relatively inexpensive to produce as compared to mAbs, as they can be produced by immunizing animals such as rabbits, goats, or mice, with the target antigen that would stimulate a polyclonal antibody response. In addition, the polyclonal antibody used in applications such as ELISA, Western Blot, and many others, are less likely to produce false negatives due to their ability to recognize multiple epitopes on the targeted antigen.
Biomedical research is projected to show significant growth during 2023-2032. This growth is due to their versatile nature, easy producibility, diverse range of binding specificities, and the ability to detect a wide range of biomolecules. In addition, as per the article published in January 2023 by ScienceDaily, scientists have developed a machine learning approach using AI technology to accelerate the production of new highly specific antibody drugs against diseases such as cancer, rheumatoid arthritis, and COVID-19. Additionally, in June 2021, Rapid Novor designed the first-of-its-kind sequencing technology, namely, REpAb, used for identifying sequences in pAbs utilized during drug discovery processes. Such technological advancements are expected to increase the demand for biomedical research during the forecast period.
Source Insights
The rabbit segment dominated the market in 2022 with a market share of 51% due to rapid immune response against small molecules, haptens, and post-translational modifications. Moreover, rabbit Abs have better affinity and specificity as compared to other animals. Rabbit-sourced antibodies (Abs) are highly specific Abs with a binding capability to various proteins in the picomolar range. Abs sourced from rabbits are highly particular, and showcase an improved immunologic response, thus facilitating better recognition of diseases during diagnosis. For instance, in February 2023, Roche announced the launch of two novel Abs (i.e., Rabbit Monoclonal Primary Antibody and the ATRX Rabbit Polyclonal Antibody) that would be used to identify the mutated gene in patients with brain cancer.
Mouse is expected to grow with the fastest CAGR of 8.05% during 2023-2032. Mice are widely available in research laboratories compared to other animals for the production of Abs that are manufactured using immunization protocols. Moreover, the growth factors for mouse-derived pAbs, include their high degree of homology with human proteins. Mice share a high degree of genetic and protein sequences similar to humans, making them one of the ideal hosts for generating pAbs that cross-react with human proteins.
End-User Insights
In 2022, the hospitals & diagnostic centers segment dominated with a market share of 60%, and the same is anticipated to have the highest CAGR rate of 5.68% during 2023-2032. Due to the rising prevalence of chronic & infectious diseases, growing cases of cancer, and increasing demand for pAbs-based assays in diagnostic centers the demand for hospitals & diagnostic centers are anticipated to increase during the forecast period. Furthermore, growing investments in hospitals & diagnostic centers are expected to boost the growth of the segment.For instance, in April 2020, the University of Arizona Health Sciences associated with the State of Arizona accompanied an antibody blood examination for the COVID-19 pandemic. To the university, the State offered financial assistance of USD 3.5 million. Hence, increasing demand for antibody-based tests is further expected to grow the demand for pAbs.
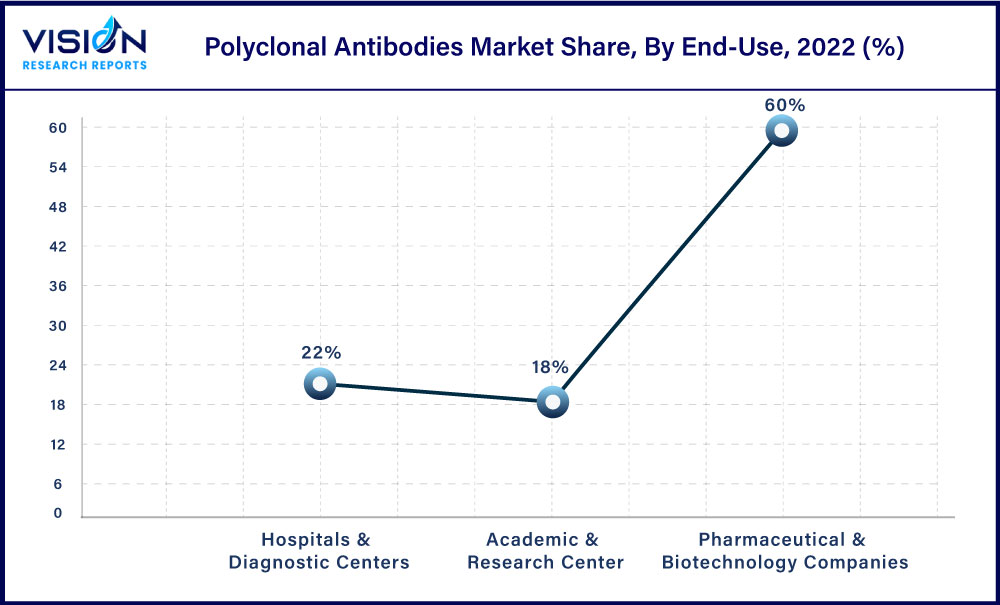
The pharmaceutical & biotechnology companies’ segment is projected to show significant growth over the forecast period owing to the increasing demand for pAbs in drug discovery & development, and growing investment in biologics. For instance, in January 2023, SAB Biotherapeutics, a clinical-stage biopharmaceutical firm announced results from a project in collaboration with global biotechnology leader CSL, approving that SAB’s DiversitAb platform can produce efficient fully-human anti-idiotype pAbs that can efficiently target and nullify autoantibodies related with autoimmune ailments.
Regional Insights
In 2022, North America dominated the industry with a market share of 39% in terms of revenue. This large market share is attributed due to several factors, including, the presence of a large number of biotechnology and pharmaceutical companies, advanced technology and infrastructure, a strong focus on innovation, a stringent regulatory environment, and a well-developed healthcare system. In July 2022, GigaGen, headquartered in California, U.S., published a study showcasing the First-Ever Clinical GMP Manufacturing and IND-Enabling Studies for the organization’s novel class of recombinant pAbs drug, an approach to treat infectious and chronic diseases, by targeting numerous viral epitopes.
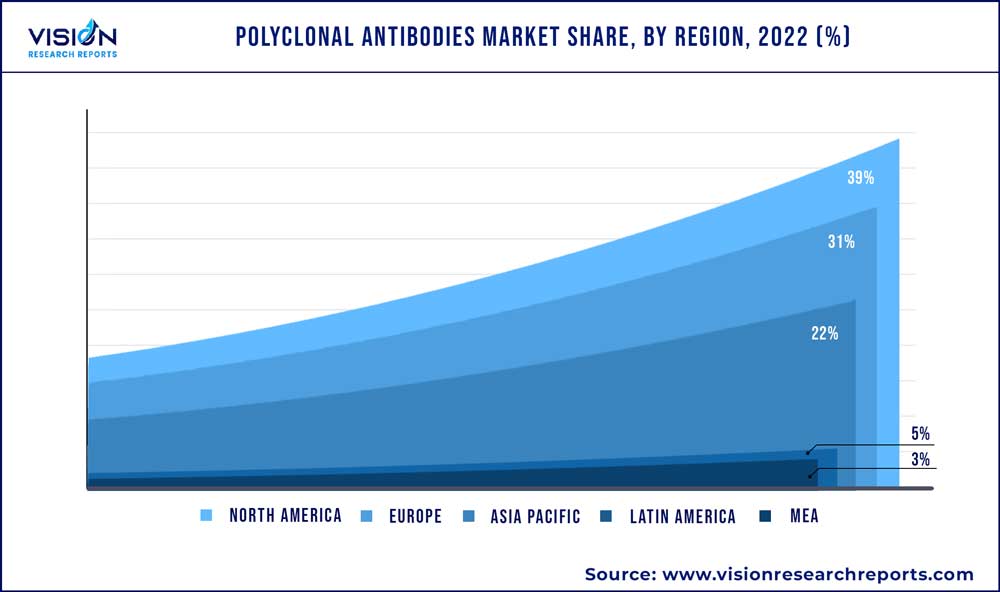
Asia Pacific is anticipated to register the highest CAGR of 9.69% during 2023-2032. The pAbs market is driven by several factors, including the increasing prevalence of chronic diseases, rising demand for personalized medicine, growing investments in research and development, and advancements in healthcare infrastructure in countries like India, China, and South Korea. As a result, the pAbs market in the Asia Pacific region is expected to continue to grow rapidly in the coming years. For instance, in May 2020, China-based biotechnology company Junshi Biosciences, collaborated with Eli Lilly and Company to jointly create therapeutic antibodies to prevent and treat illness caused due to the COVID-19 pandemic.
Polyclonal Antibodies Market Segmentations:
By Product
By Application
By Source
By End-User
By Regional
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Polyclonal Antibodies Market
5.1. COVID-19 Landscape: Polyclonal Antibodies Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Polyclonal Antibodies Market, By Product
8.1. Polyclonal Antibodies Market, by Product, 2023-2032
8.1.1. Primary Antibodies
8.1.1.1. Market Revenue and Forecast (2020-2032)
8.1.2. Secondary Antibodies
8.1.2.1. Market Revenue and Forecast (2020-2032)
Chapter 9. Global Polyclonal Antibodies Market, By Application
9.1. Polyclonal Antibodies Market, by Application, 2023-2032
9.1.1. Biomedical Research
9.1.1.1. Market Revenue and Forecast (2020-2032)
9.1.2. Diagnostics
9.1.2.1. Market Revenue and Forecast (2020-2032)
Chapter 10. Global Polyclonal Antibodies Market, By Source
10.1. Polyclonal Antibodies Market, by Source, 2023-2032
10.1.1. Rabbits
10.1.1.1. Market Revenue and Forecast (2020-2032)
10.1.2. Goats
10.1.2.1. Market Revenue and Forecast (2020-2032)
10.1.3. Sheep
10.1.3.1. Market Revenue and Forecast (2020-2032)
10.1.4. Mouse
10.1.4.1. Market Revenue and Forecast (2020-2032)
10.1.5. Others
10.1.5.1. Market Revenue and Forecast (2020-2032)
Chapter 11. Global Polyclonal Antibodies Market, By End-User
11.1. Polyclonal Antibodies Market, by End-User, 2023-2032
11.1.1. Pharmaceutical & Biotechnology Companies
11.1.1.1. Market Revenue and Forecast (2020-2032)
11.1.2. Hospitals & Diagnostic Centers
11.1.2.1. Market Revenue and Forecast (2020-2032)
11.1.3. Academic & Research Center
11.1.3.1. Market Revenue and Forecast (2020-2032)
Chapter 12. Global Polyclonal Antibodies Market, Regional Estimates and Trend Forecast
12.1. North America
12.1.1. Market Revenue and Forecast, by Product (2020-2032)
12.1.2. Market Revenue and Forecast, by Application (2020-2032)
12.1.3. Market Revenue and Forecast, by Source (2020-2032)
12.1.4. Market Revenue and Forecast, by End-User (2020-2032)
12.1.5. U.S.
12.1.5.1. Market Revenue and Forecast, by Product (2020-2032)
12.1.5.2. Market Revenue and Forecast, by Application (2020-2032)
12.1.5.3. Market Revenue and Forecast, by Source (2020-2032)
12.1.5.4. Market Revenue and Forecast, by End-User (2020-2032)
12.1.6. Rest of North America
12.1.6.1. Market Revenue and Forecast, by Product (2020-2032)
12.1.6.2. Market Revenue and Forecast, by Application (2020-2032)
12.1.6.3. Market Revenue and Forecast, by Source (2020-2032)
12.1.6.4. Market Revenue and Forecast, by End-User (2020-2032)
12.2. Europe
12.2.1. Market Revenue and Forecast, by Product (2020-2032)
12.2.2. Market Revenue and Forecast, by Application (2020-2032)
12.2.3. Market Revenue and Forecast, by Source (2020-2032)
12.2.4. Market Revenue and Forecast, by End-User (2020-2032)
12.2.5. UK
12.2.5.1. Market Revenue and Forecast, by Product (2020-2032)
12.2.5.2. Market Revenue and Forecast, by Application (2020-2032)
12.2.5.3. Market Revenue and Forecast, by Source (2020-2032)
12.2.5.4. Market Revenue and Forecast, by End-User (2020-2032)
12.2.6. Germany
12.2.6.1. Market Revenue and Forecast, by Product (2020-2032)
12.2.6.2. Market Revenue and Forecast, by Application (2020-2032)
12.2.6.3. Market Revenue and Forecast, by Source (2020-2032)
12.2.6.4. Market Revenue and Forecast, by End-User (2020-2032)
12.2.7. France
12.2.7.1. Market Revenue and Forecast, by Product (2020-2032)
12.2.7.2. Market Revenue and Forecast, by Application (2020-2032)
12.2.7.3. Market Revenue and Forecast, by Source (2020-2032)
12.2.7.4. Market Revenue and Forecast, by End-User (2020-2032)
12.2.8. Rest of Europe
12.2.8.1. Market Revenue and Forecast, by Product (2020-2032)
12.2.8.2. Market Revenue and Forecast, by Application (2020-2032)
12.2.8.3. Market Revenue and Forecast, by Source (2020-2032)
12.2.8.4. Market Revenue and Forecast, by End-User (2020-2032)
12.3. APAC
12.3.1. Market Revenue and Forecast, by Product (2020-2032)
12.3.2. Market Revenue and Forecast, by Application (2020-2032)
12.3.3. Market Revenue and Forecast, by Source (2020-2032)
12.3.4. Market Revenue and Forecast, by End-User (2020-2032)
12.3.5. India
12.3.5.1. Market Revenue and Forecast, by Product (2020-2032)
12.3.5.2. Market Revenue and Forecast, by Application (2020-2032)
12.3.5.3. Market Revenue and Forecast, by Source (2020-2032)
12.3.5.4. Market Revenue and Forecast, by End-User (2020-2032)
12.3.6. China
12.3.6.1. Market Revenue and Forecast, by Product (2020-2032)
12.3.6.2. Market Revenue and Forecast, by Application (2020-2032)
12.3.6.3. Market Revenue and Forecast, by Source (2020-2032)
12.3.6.4. Market Revenue and Forecast, by End-User (2020-2032)
12.3.7. Japan
12.3.7.1. Market Revenue and Forecast, by Product (2020-2032)
12.3.7.2. Market Revenue and Forecast, by Application (2020-2032)
12.3.7.3. Market Revenue and Forecast, by Source (2020-2032)
12.3.7.4. Market Revenue and Forecast, by End-User (2020-2032)
12.3.8. Rest of APAC
12.3.8.1. Market Revenue and Forecast, by Product (2020-2032)
12.3.8.2. Market Revenue and Forecast, by Application (2020-2032)
12.3.8.3. Market Revenue and Forecast, by Source (2020-2032)
12.3.8.4. Market Revenue and Forecast, by End-User (2020-2032)
12.4. MEA
12.4.1. Market Revenue and Forecast, by Product (2020-2032)
12.4.2. Market Revenue and Forecast, by Application (2020-2032)
12.4.3. Market Revenue and Forecast, by Source (2020-2032)
12.4.4. Market Revenue and Forecast, by End-User (2020-2032)
12.4.5. GCC
12.4.5.1. Market Revenue and Forecast, by Product (2020-2032)
12.4.5.2. Market Revenue and Forecast, by Application (2020-2032)
12.4.5.3. Market Revenue and Forecast, by Source (2020-2032)
12.4.5.4. Market Revenue and Forecast, by End-User (2020-2032)
12.4.6. North Africa
12.4.6.1. Market Revenue and Forecast, by Product (2020-2032)
12.4.6.2. Market Revenue and Forecast, by Application (2020-2032)
12.4.6.3. Market Revenue and Forecast, by Source (2020-2032)
12.4.6.4. Market Revenue and Forecast, by End-User (2020-2032)
12.4.7. South Africa
12.4.7.1. Market Revenue and Forecast, by Product (2020-2032)
12.4.7.2. Market Revenue and Forecast, by Application (2020-2032)
12.4.7.3. Market Revenue and Forecast, by Source (2020-2032)
12.4.7.4. Market Revenue and Forecast, by End-User (2020-2032)
12.4.8. Rest of MEA
12.4.8.1. Market Revenue and Forecast, by Product (2020-2032)
12.4.8.2. Market Revenue and Forecast, by Application (2020-2032)
12.4.8.3. Market Revenue and Forecast, by Source (2020-2032)
12.4.8.4. Market Revenue and Forecast, by End-User (2020-2032)
12.5. Latin America
12.5.1. Market Revenue and Forecast, by Product (2020-2032)
12.5.2. Market Revenue and Forecast, by Application (2020-2032)
12.5.3. Market Revenue and Forecast, by Source (2020-2032)
12.5.4. Market Revenue and Forecast, by End-User (2020-2032)
12.5.5. Brazil
12.5.5.1. Market Revenue and Forecast, by Product (2020-2032)
12.5.5.2. Market Revenue and Forecast, by Application (2020-2032)
12.5.5.3. Market Revenue and Forecast, by Source (2020-2032)
12.5.5.4. Market Revenue and Forecast, by End-User (2020-2032)
12.5.6. Rest of LATAM
12.5.6.1. Market Revenue and Forecast, by Product (2020-2032)
12.5.6.2. Market Revenue and Forecast, by Application (2020-2032)
12.5.6.3. Market Revenue and Forecast, by Source (2020-2032)
12.5.6.4. Market Revenue and Forecast, by End-User (2020-2032)
Chapter 13. Company Profiles
13.1. Thermo Fisher Scientific Inc.
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. Merck KgaA
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. Abcam plc.
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. ProteoGenix
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. Proteintech Group, Inc.
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. Bio-Rad Laboratories Inc.
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. BPS Bioscience, Inc.
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. R&D Systems, Inc.
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. Agilent Technologies, Inc.
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. Atlas Antibodies
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others