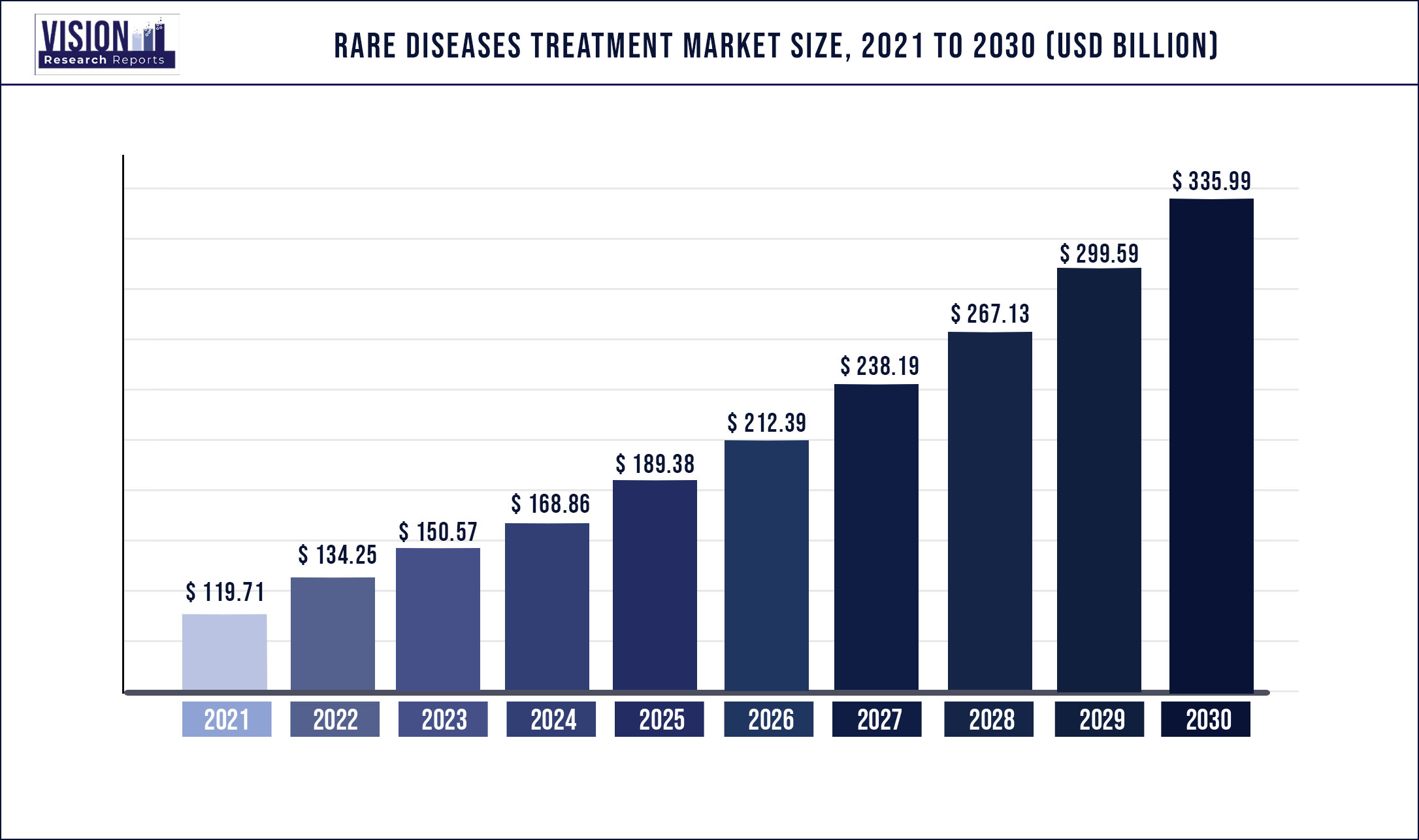
The global rare diseases treatment market was surpassed at USD 119.71 billion in 2021 and is expected to hit around USD 335.99 billion by 2030, growing at a CAGR of 12.15% from 2022 to 2030
Report Highlights
Increasing prevalence of rare diseases contributes to industry growth. According to the National Center for Advancing Translational Sciences (NCATS), over 30 million of the U.S. population is estimated to be affected by rare diseases in 2022. Increasing prevalence of people living with orphan diseases is anticipated to increase the sales and demand for orphan medicines over the forecast period.
The presence of supportive regulations by government authorities, such as the Orphan Drug Act that support product development by offering the orphan drug designation to potential drug candidates developed by pharmaceutical companies, is expected to support industry growth. This initiative boosts the research and development in the field of orphan disease treatment.
In April 2020, the National Organization for Rare Disorders (NORD) launched the “COVID-19 critical relief” program for patients with rare diseases affected by the COVID-19 pandemic. Under this program, the NORD provided monetary assistance of up to USD 1,000 annually to orphan disease patients and their caregivers for fulfilling their medical and non-medical needs. Thus, the availability of such programs is anticipated to boost the adoption of rare disease treatment over the forecast period.
Moreover, companies have undertaken initiatives such as collaborations and partnerships for the development, manufacturing, and commercialization of products in the international market. For instance, in August 2020, Sarepta Therapeutics, Inc. entered into a collaboration with the University of Florida for the development of novel genetic medicines for the treatment of patients with various orphan diseases, including Duchenne Muscular Dystrophy (DMD).
However, a lack of accurate and early diagnosis of rare diseases can often present a challenge for orphan disease patients, and in some cases, an accurate diagnosis is not obtained for as long as five years. In addition, patient eligibility and recruitment of eligible patients for clinical trials for rare disease treatment may restrain the industry growth. Not only finding the target population is difficult but finding physicians who treat these patients is also complex, thus leading to delays in the new product approval and launch in the market.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 119.71 billion |
| Revenue Forecast by 2030 | USD 335.99 billion |
| Growth rate from 2022 to 2030 | CAGR of 12.15% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Therapeutic area, route of administration, drug type, distribution channel, region |
| Companies Covered | F. Hoffmann-La Roche Ltd.; Pfizer, Inc.; PTC Therapeutics; AstraZeneca; Novartis AG; Novo Nordisk; Bayer AG; AbbVie Inc.; Merck & Co. Inc.; Bristol Myers Squibb |
Therapeutic Area Insights
Cancer dominated the market in 2021 with a revenue share of over 25.06%. This dominance can be attributed to the high prevalence and recurrence rate of rare cancer indications. According to the American Cancer Society, the estimated incidence of esophageal cancer, chronic myeloid leukemia, and anal cancer was found to be 19,260, 9,110, and 9,090 respectively in the U.S. in 2021. Based on the therapeutic area, the global market is segmented into cancer, neurological conditions, cardiovascular conditions, musculoskeletal conditions, hematologic disorders, infectious diseases, metabolic disorders, endocrine disorders, and others.
Musculoskeletal condition is expected to be the fastest-growing segment during the projection period due to the increasing prevalence of this condition and new product approvals for its treatment. For instance, in March 2020, NS Pharma’s VILTEPSO candidate was approved in Japan for the treatment of patients with Duchenne muscular dystrophy (DMD). It was granted the SAKIGAKE designation by the healthcare body of Japan. This approval is also expected to boost segment growth. Osteogenesis imperfecta, achondroplasia, and fibrous dysplasia are some of the rare musculoskeletal conditions found in patients.
Route Of Administration Insights
The injectable segment dominated the market with a revenue share of over 50.11% in 2021 and is expected to witness significant growth during the forecast period. This can be attributed to the launch of novel injectables for the treatment of rare diseases. For instance, in February 2021, the U.S. FDA approved Sarepta Therapeutics’ AMONDYS 45 (casimersen injection) for the treatment of DMD. Based on route of administration, the global market is segmented into oral, injectable, and others.
Recently, in July 2022, Pfizer Inc. received approval for Xalkori (crizotinib) by the U.S. FDA for the treatment of pediatric and adult patients with ALK-positive inflammatory myofibroblastic tumor (IMT). The recommended dose in adult patients is 250 mg and is to be administrated orally twice daily until disease progression stops. This approval is also expected to fuel the oral segment growth.
Drug Type Insights
Biologics dominated the market with a revenue share of over 55.21% in 2021. The advancement in biotechnology and research techniques has facilitated the development of novel biologics. High target specificity and potential of biological drugs revolutionizing the treatments of several rare diseases are expected to fuel the segment growth. For instance, in 2019, the U.S. FDA approved Zolgensma, a gene therapy, for the treatment of spinal muscular atrophy. Based on drug type, the global market is segmented into biologics, biosimilars, and small molecules.
The emergence of biosimilars for the treatment of rare diseases proved to be a breakthrough for patients. The patent expiration of orphan biologics and supportive regulatory policies will pave the way for new players to enter the market and drive competition, leading to a reduction in drug price that is used for the treatment of various rare diseases, thereby fueling the segment growth. For instance, according to the Center for Biosimilars, approximately 11% of orphan biologics providers have biosimilar candidates in the product pipeline. However, high costs associated with R&D and a small consumer base may hamper the segment growth.
Distribution Channel Insights
The specialty pharmacy segment dominated the market with a revenue share of over 70.15% in 2021. The dominance can be attributed to strategic initiatives undertaken by key players, such as the acquisition of specialty pharmacies for the distribution of their products. For instance, in December 2020, Centene acquired PANTHERx, the largest specialty pharmacy in the U.S., which engages in distributing high-cost orphan drugs that are used for the treatment of various types of rare diseases. Centene serves as an intermediary for government and privately sponsored health insurance programs. Such acquisitions are expected to boost segment growth during the forecast period. Based on distribution channels, the global market is further segmented into hospital pharmacy, specialty pharmacy, and online pharmacy.
Hospital pharmacy is expected to be the fastest-growing segment during the assessment period. This can be attributed to the high hospitalization rate of SMA. The majority of patients are treated in hospitals due to the high risk of respiratory issues and scoliosis. According to the Orphanet Journal of Rare Diseases (2020), the inpatient ratio was the highest in pediatric patients aged 0 to 4 years.
Regional Insights
North America dominated the market in 2021 with a revenue share of over 60.02% due to the high burden of diseases, favorable healthcare infrastructure, and new product approvals for treatment. In October 2021, AstraZeneca received orphan drug designation for Tezepelumab from the U.S. FDA for the treatment of eosinophilic esophagitis (EoE). The accessibility to products may increase patient compliance, consequently expanding the consumer base and increasing the revenue for the market.
The Asia Pacific is expected to be the fastest-growing region during the forecast period. The growth of the region can be attributed to the initiatives undertaken by governments to support orphan disease patients. For instance, in July 2022, the Indian government directed national and state governments to ensure the effective implantation of health policies developed for the treatment of patients suffering from orphan diseases. This measure creates an opportunity for manufacturers to supply high-quality orphan medicines to the government and generate revenue.
Key Players
Market Segmentation
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Rare Diseases Treatment Market
5.1. COVID-19 Landscape: Rare Diseases Treatment Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Rare Diseases Treatment Market, By Therapeutic Area
8.1. Rare Diseases Treatment Market, by Therapeutic Area, 2022-2030
8.1.1. Cancer
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Neurological Conditions
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Cardiovascular Conditions
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Musculoskeletal Conditions
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Hematologic Disorders
8.1.5.1. Market Revenue and Forecast (2017-2030)
8.1.6. Infectious Diseases
8.1.6.1. Market Revenue and Forecast (2017-2030)
8.1.7. Metabolic disorders
8.1.7.1. Market Revenue and Forecast (2017-2030)
8.1.8. Endocrine disorders
8.1.8.1. Market Revenue and Forecast (2017-2030)
8.1.9. Others
8.1.9.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Rare Diseases Treatment Market, By Route of Administration
9.1. Rare Diseases Treatment Market, by Route of Administration e, 2022-2030
9.1.1. Oral
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Injectable
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Others
9.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Rare Diseases Treatment Market, By Drug Type
10.1. Rare Diseases Treatment Market, by Drug Type, 2022-2030
10.1.1. Biologics
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Biosimilar
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Small Molecule
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Rare Diseases Treatment Market, By Distribution Channel
11.1. Rare Diseases Treatment Market, by Distribution Channel, 2022-2030
11.1.1. Hospital Pharmacy
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Specialty Pharmacy
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Online Pharmacy
11.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global Rare Diseases Treatment Market, Regional Estimates and Trend Forecast
12.1. North America
12.1.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.1.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.1.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.1.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.1.5. U.S.
12.1.5.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.1.5.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.1.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.1.5.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.1.6. Rest of North America
12.1.6.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.1.6.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.1.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.1.6.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.2. Europe
12.2.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.2.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.2.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.2.5. UK
12.2.5.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.2.5.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.2.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.5.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.2.6. Germany
12.2.6.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.2.6.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.2.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.6.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.2.7. France
12.2.7.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.2.7.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.2.7.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.7.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.2.8. Rest of Europe
12.2.8.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.2.8.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.2.8.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.2.8.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.3. APAC
12.3.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.3.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.3.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.3.5. India
12.3.5.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.3.5.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.3.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.5.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.3.6. China
12.3.6.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.3.6.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.3.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.6.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.3.7. Japan
12.3.7.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.3.7.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.3.7.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.7.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.3.8. Rest of APAC
12.3.8.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.3.8.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.3.8.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.3.8.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.4. MEA
12.4.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.4.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.4.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.4.5. GCC
12.4.5.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.4.5.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.4.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.5.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.4.6. North Africa
12.4.6.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.4.6.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.4.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.6.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.4.7. South Africa
12.4.7.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.4.7.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.4.7.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.7.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.4.8. Rest of MEA
12.4.8.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.4.8.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.4.8.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.4.8.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.5. Latin America
12.5.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.5.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.5.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.5.5. Brazil
12.5.5.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.5.5.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.5.5.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.5.5.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.5.6. Rest of LATAM
12.5.6.1. Market Revenue and Forecast, by Therapeutic Area (2017-2030)
12.5.6.2. Market Revenue and Forecast, by Route of Administration (2017-2030)
12.5.6.3. Market Revenue and Forecast, by Drug Type (2017-2030)
12.5.6.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
Chapter 13. Company Profiles
13.1. F. Hoffmann-La Roche Ltd.
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. Pfizer, Inc.
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. PTC Therapeutics
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. AstraZeneca
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. Novartis AG
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. Takeda Pharmaceutical Company
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. Bayer AG
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. AbbVie Inc.
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. Merck & Co. Inc.
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. Bristol Myers Squibb
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others