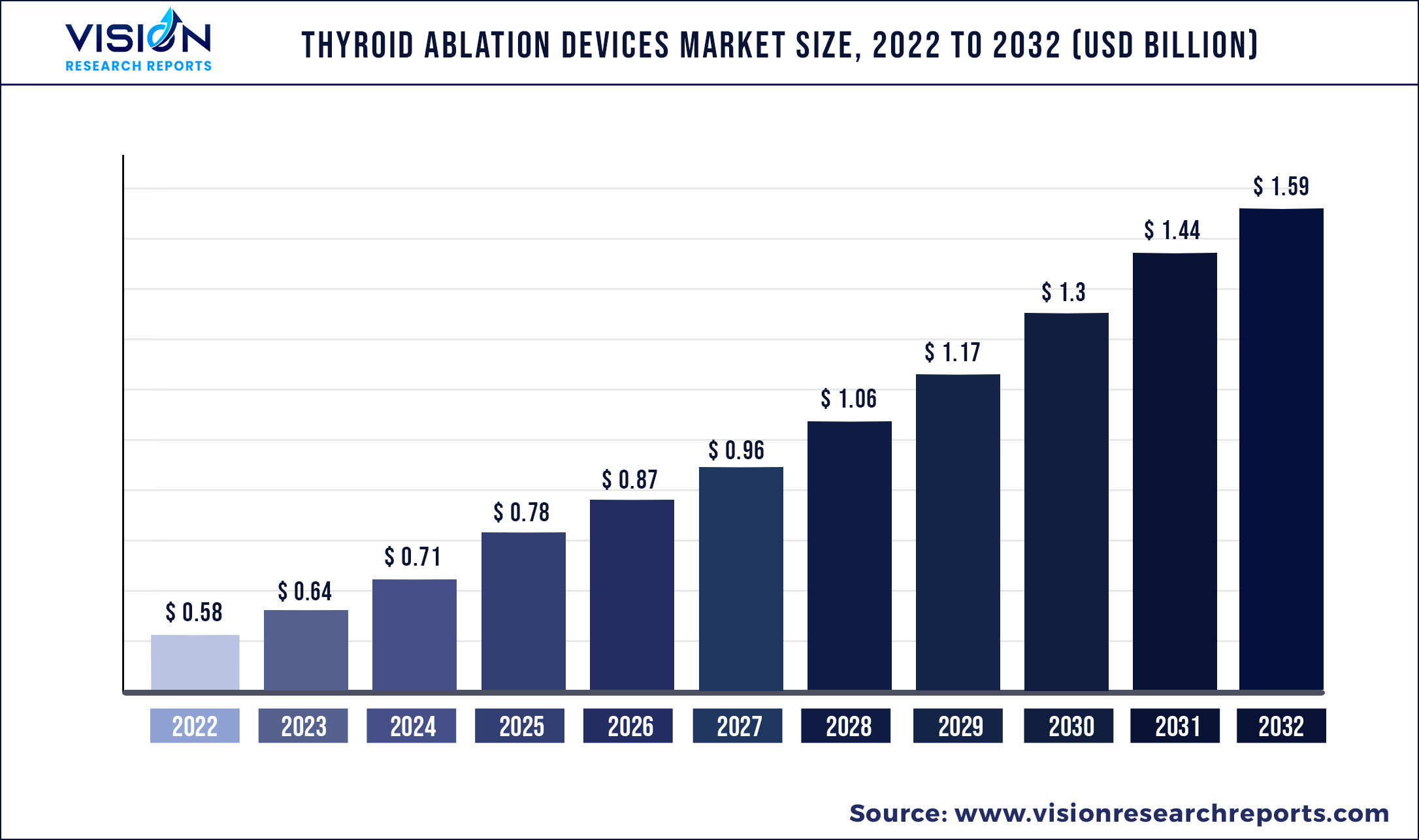
The global thyroid ablation devices market size was estimated at around USD 0.58 billion in 2022 and it is projected to hit around USD 1.59 billion by 2032, growing at a CAGR of 10.64% from 2023 to 2032.
Rising prevalence of thyroid cancer & thyroid nodules, growing preference of minimally invasive surgeries, and availability of technologically sound ablation procedures is expected to drive the thyroid ablation devices market globally. Additionally, presence of government support & funding for the treatment of Hashimoto’s thyroiditis, an autoimmune disorder that attack thyroid cells and cause hypothyroidism. Hashimoto’s thyroiditis is the most common cause of developing hypothyroidism, almost 5 out of 100 veterans suffer from it. According to NCBI, the incidence is expected to be 0.8 per 1000 per year in men and 3.5 per 1000 per year in women.
Moreover, according to American Cancer Society's (ACS) publications, around 43,800 adults among which 31,940 are females and 11,860 are males in the U.S. is expected to be diagnosed with the thyroid cancer in 2022. The seventh frequent type of cancer found in women is thyroid cancer. The cases of thyroid cancer diagnoses worldwide are estimated to reach 586,202 in 2020. Thyroid cancer is three times as common in women than in men. The estimated global death toll from thyroid cancer in 2020 is 43,646.
Thyroid ablation devices are the most frequently used for the treatment thyroid nodules and thyroid cancers. An abnormally growing mass of thyroid cells in the thyroid gland is known as a thyroid nodule. The thyroid gland is positioned close to the base of the throat, and thyroid cancer is a type of solid tumor malignancy that turns up as a nodule or lump there. It occurs when rogue cells proliferate at a rate that is faster than the immune system can cope with. Although there are many different types of thyroid cancer, papillary thyroid cancer and follicular thyroid cancer account for almost all instances (95 percent).
Thyroid cancer is known to be at increased risk due to radiation exposure. The International Agency for Research on Cancer (IARC) has found that persons who are overweight or obese are more likely than those who are not to get thyroid cancer. In regions of the world where iodine intake is low, follicular thyroid cancers are more prevalent. Iodine-rich diets, however, may increase the risk of papillary thyroid cancer. Because it is added to table salt and other meals in the United States, the majority of people consume enough iodine.
Thyroid ablation devices market has been segmented on the basis of type, product type, application, and end user. The type segment is classified into image-guided ablation, radiofrequency ablation, microwave ablation, and others. Thermal based devices and non-thermal based devices comes under the product type segment. On the basis of application, the market is bifurcated into thyroid cancer and thyroid nodules. The thyroid cancer is further segmented into its types such as papillary, follicular, medullary, and anaplastic thyroid carcinomas. The end-user segment includes hospitals, ambulatory surgical centers, cancer specialty centers, and others. Furthermore, the market is segmented on the basis on regional outlook into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
| Report Coverage | Details |
| Market Size in 2022 | USD 0.58 billion |
| Revenue Forecast by 2032 | USD 1.59 billion |
| Growth rate from 2023 to 2032 | CAGR of 10.64% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Segmentation | Type, Product Type. Application, End User, Region |
| Companies Covered | Boston Scientific Corporation, BVM Medical System, Integra Life Sciences, Johnson & Johnson, Medtronic Plc., MedWaves Inc., Olympus Corporation, StarMed Co.Ltd., Terumo Europe, and Theraclion |
The Covid-19 pandemic has impacted negatively on the global thyroid ablation devices market. The planned thyroid ablation surgeries and thyroid cancer surgeries were delayed on the onset of infection by almost 4 weeks as they were elective or non-essential surgeries. However, the maintenance and installation cost of thyroid ablation devices is expected to create a stumbling block in the market growth during forecast period.
The major players operating in the global thyroid ablation devices market are Boston Scientific Corporation, BVM Medical System, Integra Life Sciences, Johnson & Johnson, Medtronic Plc., MedWaves Inc., Olympus Corporation, StarMed Co.Ltd., Terumo Europe, and Theraclion. Moroever, companies are involved in various strategic initiatives in order to fuel the growth of the market. For Instance, Ethicon, a subsidiary of Johnson & Johnson has received FDA breakthrough device designation for their robot-assisted bronchoscopy-based transbronchial microwave ablation technique in 2020. Additionally, In August 2020, the QDOT MICRO radiofrequency (RF) ablation catheter from Biosense Webster, a subsidiary of Johnson & Johnson Medical Devices Companies, has been given European CE mark approval. The usage of the device is approved in both Europe and Japan. In the US, there is still an investigation going on.
Market Segmentation
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Thyroid Ablation Devices Market
5.1. COVID-19 Landscape: Thyroid Ablation Devices Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Thyroid Ablation Devices Market, By Type
8.1. Thyroid Ablation Devices Market, by Type, 2023-2032
8.1.1. Image-guided Ablation
8.1.1.1. Market Revenue and Forecast (2019-2032)
8.1.2. Radiofrequency Ablation
8.1.2.1. Market Revenue and Forecast (2019-2032)
8.1.3. Microwave Ablation
8.1.3.1. Market Revenue and Forecast (2019-2032)
8.1.4. Others
8.1.4.1. Market Revenue and Forecast (2019-2032)
Chapter 9. Global Thyroid Ablation Devices Market, By Product Type
9.1. Thyroid Ablation Devices Market, by Product Type, 2023-2032
9.1.1. Thermal Based Devices
9.1.1.1. Market Revenue and Forecast (2019-2032)
9.1.2. Non-Thermal Based Devices
9.1.2.1. Market Revenue and Forecast (2019-2032)
Chapter 10. Global Thyroid Ablation Devices Market, By Application
10.1. Thyroid Ablation Devices Market, by Application, 2023-2032
10.1.1. Thyroid Cancer
10.1.1.1. Market Revenue and Forecast (2019-2032)
10.1.2. Thyroid Nodules
10.1.2.1. Market Revenue and Forecast (2019-2032)
Chapter 11. Global Thyroid Ablation Devices Market, By End User
11.1. Thyroid Ablation Devices Market, by End User, 2023-2032
11.1.1. Hospitals
11.1.1.1. Market Revenue and Forecast (2019-2032)
11.1.2. Ambulatory Surgical Centers
11.1.2.1. Market Revenue and Forecast (2019-2032)
11.1.3. Cancer Specialty Centers
11.1.3.1. Market Revenue and Forecast (2019-2032)
11.1.4. Others
11.1.4.1. Market Revenue and Forecast (2019-2032)
Chapter 12. Global Thyroid Ablation Devices Market, Regional Estimates and Trend Forecast
12.1. North America
12.1.1. Market Revenue and Forecast, by Type (2019-2032)
12.1.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.1.3. Market Revenue and Forecast, by Application (2019-2032)
12.1.4. Market Revenue and Forecast, by End User (2019-2032)
12.1.5. U.S.
12.1.5.1. Market Revenue and Forecast, by Type (2019-2032)
12.1.5.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.1.5.3. Market Revenue and Forecast, by Application (2019-2032)
12.1.5.4. Market Revenue and Forecast, by End User (2019-2032)
12.1.6. Rest of North America
12.1.6.1. Market Revenue and Forecast, by Type (2019-2032)
12.1.6.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.1.6.3. Market Revenue and Forecast, by Application (2019-2032)
12.1.6.4. Market Revenue and Forecast, by End User (2019-2032)
12.2. Europe
12.2.1. Market Revenue and Forecast, by Type (2019-2032)
12.2.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.2.3. Market Revenue and Forecast, by Application (2019-2032)
12.2.4. Market Revenue and Forecast, by End User (2019-2032)
12.2.5. UK
12.2.5.1. Market Revenue and Forecast, by Type (2019-2032)
12.2.5.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.2.5.3. Market Revenue and Forecast, by Application (2019-2032)
12.2.5.4. Market Revenue and Forecast, by End User (2019-2032)
12.2.6. Germany
12.2.6.1. Market Revenue and Forecast, by Type (2019-2032)
12.2.6.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.2.6.3. Market Revenue and Forecast, by Application (2019-2032)
12.2.6.4. Market Revenue and Forecast, by End User (2019-2032)
12.2.7. France
12.2.7.1. Market Revenue and Forecast, by Type (2019-2032)
12.2.7.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.2.7.3. Market Revenue and Forecast, by Application (2019-2032)
12.2.7.4. Market Revenue and Forecast, by End User (2019-2032)
12.2.8. Rest of Europe
12.2.8.1. Market Revenue and Forecast, by Type (2019-2032)
12.2.8.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.2.8.3. Market Revenue and Forecast, by Application (2019-2032)
12.2.8.4. Market Revenue and Forecast, by End User (2019-2032)
12.3. APAC
12.3.1. Market Revenue and Forecast, by Type (2019-2032)
12.3.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.3.3. Market Revenue and Forecast, by Application (2019-2032)
12.3.4. Market Revenue and Forecast, by End User (2019-2032)
12.3.5. India
12.3.5.1. Market Revenue and Forecast, by Type (2019-2032)
12.3.5.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.3.5.3. Market Revenue and Forecast, by Application (2019-2032)
12.3.5.4. Market Revenue and Forecast, by End User (2019-2032)
12.3.6. China
12.3.6.1. Market Revenue and Forecast, by Type (2019-2032)
12.3.6.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.3.6.3. Market Revenue and Forecast, by Application (2019-2032)
12.3.6.4. Market Revenue and Forecast, by End User (2019-2032)
12.3.7. Japan
12.3.7.1. Market Revenue and Forecast, by Type (2019-2032)
12.3.7.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.3.7.3. Market Revenue and Forecast, by Application (2019-2032)
12.3.7.4. Market Revenue and Forecast, by End User (2019-2032)
12.3.8. Rest of APAC
12.3.8.1. Market Revenue and Forecast, by Type (2019-2032)
12.3.8.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.3.8.3. Market Revenue and Forecast, by Application (2019-2032)
12.3.8.4. Market Revenue and Forecast, by End User (2019-2032)
12.4. MEA
12.4.1. Market Revenue and Forecast, by Type (2019-2032)
12.4.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.4.3. Market Revenue and Forecast, by Application (2019-2032)
12.4.4. Market Revenue and Forecast, by End User (2019-2032)
12.4.5. GCC
12.4.5.1. Market Revenue and Forecast, by Type (2019-2032)
12.4.5.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.4.5.3. Market Revenue and Forecast, by Application (2019-2032)
12.4.5.4. Market Revenue and Forecast, by End User (2019-2032)
12.4.6. North Africa
12.4.6.1. Market Revenue and Forecast, by Type (2019-2032)
12.4.6.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.4.6.3. Market Revenue and Forecast, by Application (2019-2032)
12.4.6.4. Market Revenue and Forecast, by End User (2019-2032)
12.4.7. South Africa
12.4.7.1. Market Revenue and Forecast, by Type (2019-2032)
12.4.7.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.4.7.3. Market Revenue and Forecast, by Application (2019-2032)
12.4.7.4. Market Revenue and Forecast, by End User (2019-2032)
12.4.8. Rest of MEA
12.4.8.1. Market Revenue and Forecast, by Type (2019-2032)
12.4.8.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.4.8.3. Market Revenue and Forecast, by Application (2019-2032)
12.4.8.4. Market Revenue and Forecast, by End User (2019-2032)
12.5. Latin America
12.5.1. Market Revenue and Forecast, by Type (2019-2032)
12.5.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.5.3. Market Revenue and Forecast, by Application (2019-2032)
12.5.4. Market Revenue and Forecast, by End User (2019-2032)
12.5.5. Brazil
12.5.5.1. Market Revenue and Forecast, by Type (2019-2032)
12.5.5.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.5.5.3. Market Revenue and Forecast, by Application (2019-2032)
12.5.5.4. Market Revenue and Forecast, by End User (2019-2032)
12.5.6. Rest of LATAM
12.5.6.1. Market Revenue and Forecast, by Type (2019-2032)
12.5.6.2. Market Revenue and Forecast, by Product Type (2019-2032)
12.5.6.3. Market Revenue and Forecast, by Application (2019-2032)
12.5.6.4. Market Revenue and Forecast, by End User (2019-2032)
Chapter 13. Company Profiles
13.1. Boston Scientific Corporation
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. BVM Medical System
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. Integra Life Sciences
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. Johnson & Johnson
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. Medtronic Plc.
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. MedWaves Inc.
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. Olympus Corporation
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. StarMed Co.Ltd.
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. Terumo Europe
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. Theraclion
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others