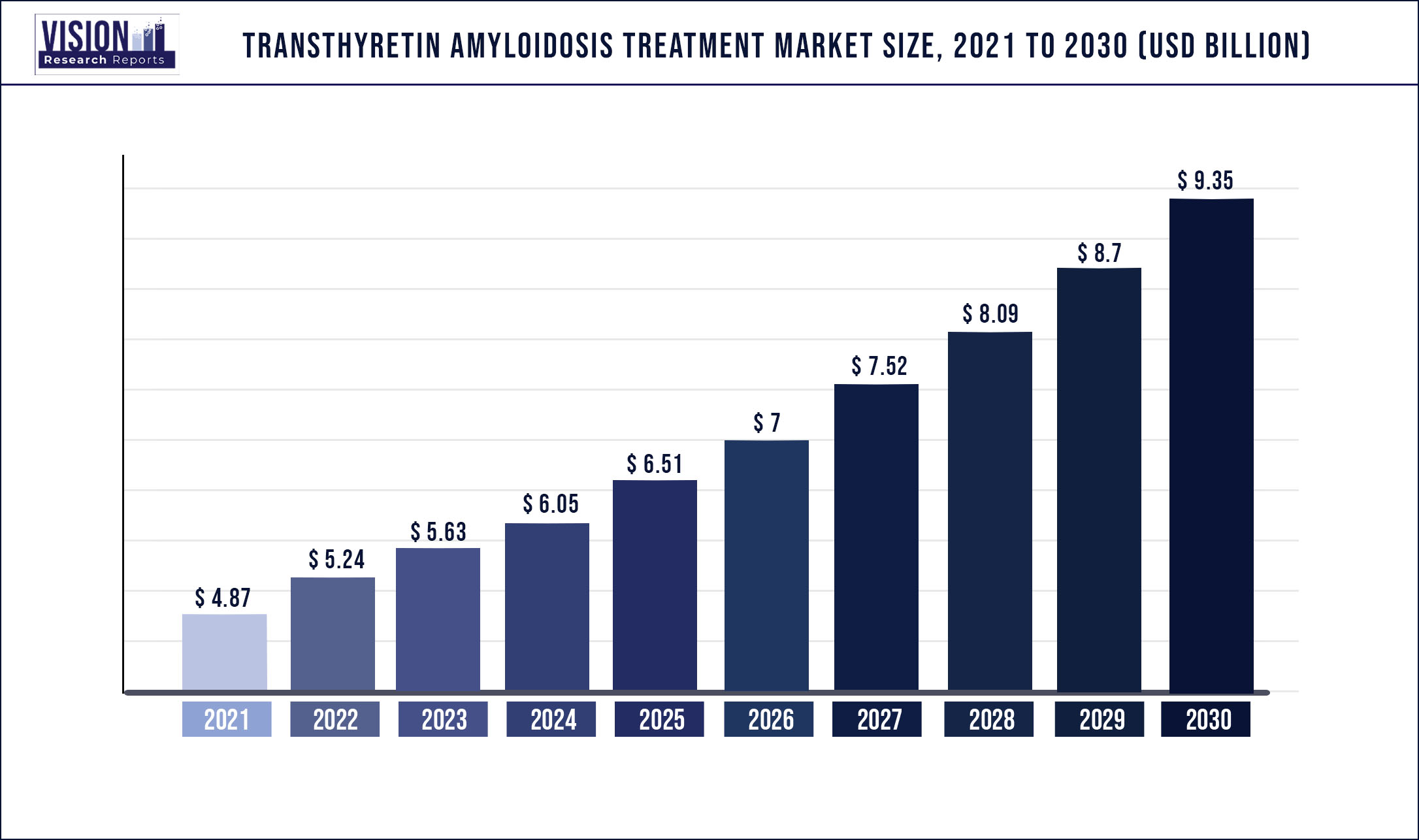
The global transthyretin amyloidosis treatment market size was estimated at around USD 4.87 billion in 2021 and it is projected to hit around USD 9.35 billion by 2030, growing at a CAGR of 7.52% from 2022 to 2030.
Report Highlights
The transthyretin amyloidosis treatment market is mainly driven by the presence of supportive reimbursement programs and the growing African American population. The increasing African American population acted as a catalyst for the industry growth throughout the period owing to the high susceptibility to ATTR amyloidosis. The Val122Ile is the most prevalent mutation worldwide that leads to cardiomyopathy among the population specific to aged 65 years and above. Furthermore, this Val122Ile mutation is present in 3% to 4% of the African American population worldwide.
An increase in research activities for the development of novel diagnostic techniques and multidisciplinary therapeutic approaches is expected to lead to an increased survival rate and a more favorable prognosis of transthyretin amyloidosis. For instance, in November 2019, the Amyloidosis Research Consortium developed a disease-specific patient-reported outcome (PRO) tool to understand and measure the impact of transthyretin amyloidosis from the patient perspective. An introduction of such a tool will boost the research in developing a new target-specific drug by understanding disease complexity and patients’ need, thereby widening the opportunity in the transthyretin amyloidosis treatment market.
Strategic initiatives undertaken by major players such as creating awareness regarding the target conditions such as ATTR and the long-term benefits of available therapies are expected to fuel industry growth. For instance, in 2019, Pfizer, Inc., in collaboration with the World Heart Federation, started the “Heart Hero” campaign. Under this campaign, Pfizer, Inc. provides educational information and resources related to ATTR-CM to patients and physicians. Such campaigns are expected to boost the early diagnosis of the disease and increase the treatment rate over time.
The movement undertaken by key players to provide free-of-cost medicine under their CSR activities is also projected to fuel market growth. For instance, the Pfizer Patient Assistance Program provides Pfizer medicines such as VYNDAMAX free of cost to eligible patients. People diagnosed with amyloidosis can take advantage of such programs by enrolling at the Vandalink platform. Such programs are expected to encourage physicians to prescribe this drug to a patient as drug therapy in transthyretin amyloidosis patients.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 4.87 billion |
| Revenue Forecast by 2030 | USD 9.35 billion |
| Growth rate from 2022 to 2030 | CAGR of 7.52% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Type, therapy, disease type, distribution channel, region |
| Companies Covered |
Pfizer, Inc.; Johnson & Johnson Services, Inc.; Ionis Pharmaceuticals, Inc.; Alnylam Pharmaceuticals, Inc.; BridgeBio Pharma, Inc.; Bristol-Myers Squibb Company; Acrotech Biopharma; AstraZeneca; Prothena; SOM Biotech |
Type Insights
ATTR-CM dominated the market for transthyretin amyloidosis and accounted for a revenue share of more than 80.11% in 2021. This is owing to the high prevalence of disease and improving diagnosis rates at a significant pace. The exact prevalence of the disease is unknown, but various autopsy studies suggest that around 22% to 25% of people aged 80 years and above reported TTR amyloid deposition in the heart. Although in most cases, the degree of amyloid deposition is mild among ATTR-CM amyloidosis patients. Moreover, the launch of new drugs for the treatment of ATTR-CM is expected to boost segment growth. For instance, in May 2019, Pfizer Inc. received approval for its VYNDAQEL and VYNDAMAX from the U.S. FDA for the treatment of ATTR-CM.
ATTR-PN is estimated to register the highest growth rate over the forecast period. The growth of the segment is attributed to the high incidence rate and approval of new drug therapies for the treatment of ATTR-PN amyloidosis patients. Val30Met is the most common TTR mutation found in patients with neuropathy. Inotersen and Patisiran are the two FDA-approved drugs available for the treatment of ATTR-PN in the market. Both drugs inhibit the production of TTR in the liver and have shown to be effective in ATTR-PN. Furthermore, the fast-track approval of products worldwide is expected to boost segment growth. For instance, in June 2019, the Ministry of Health, Labor, and Welfare of Japan approved ONPATTRO (Patisiran) for the treatment of hATTR in adults.
Disease Type Insights
Hereditary transthyretin amyloidosis captured the largest revenue share of over 60.21% in 2021. Hereditary transthyretin-mediated amyloidosis is an autosomal dominant, rare, and fatal disease in which it impairs multiple organs, leading to death and disability. To overcome this situation, pharmaceutical companies are constantly working on drug development and several drugs are still in pipeline for commercialization. For instance, in August 2022, Attralus, Inc. received U.S. FDA authorization of orphan drug designation for 124I-AT-01, which could be used for the diagnosis of transthyretin amyloidosis.
Furthermore, several countries are anticipated to witness a higher number of cardiomyopathy cases. Therefore, intensive R&D is happening in the market for the proper diagnosis and treatment of the disease. For instance, in August 2022, Alnylam Pharmaceuticals, Inc. received FDA authorization for the drug Onpattro, which could be used for nerve pain treatment caused by hereditary transthyretin amyloidosis. Moreover, its phase 3 data shows that it has the potential to help patients suffering from cardiac-related issues for rare protein diseases.
Distribution Channel Insights
Hospital pharmacies captured the largest revenue share of over 30.08% in 2021. Most hospitals are working toward the diagnosis, research, and treatment of the disease. For instance, Brigham and Women’s Hospital in Massachusetts, U.S. focuses on diagnosing and treating misdiagnosed diseases. It has established the Amyloidosis Program, which offers access to innovative therapies, patient-centered care, and new clinical trials. The team of experts would educate physicians and patients and spread awareness among the patients. These initiatives would further contribute to the segment's growth.
Online pharmacies are anticipated to witness the fastest growth in the forecast period owing to the easy access of drugs to patients, which is also expanding the use to get health information. However, there is an increasing risk of patients purchasing products from illegal websites is expected to hamper the growth. For instance, an online publication stated that fraudulent online pharmacies are attempting to sell illegal generic versions of Vyndamax. This could be potentially unsafe for the customers. Therefore, promotional campaigns would further spread awareness among the public about safe and authentic online pharmacies, which could further prevent safety threats to patients.
Therapy Insights
The targeted therapy segment dominated the market and accounted for a revenue share of over 90.17% in 2021. Drugs such as Onpattro, Inotersen, and Tafamidis are included in this segment. Onpattro is administrated intravenously to ATTR-PN patients at an interval of 3 weeks with pretreatment of steroids and Benadryl to avoid side effects. Onpattro works by silencing the portion of RNA involved in producing TTR protein. Moreover, an increasing prevalence of the disease ATTR-PN and worldwide approval of these therapies are projected to fuel segment growth. For instance, in July 2018, the Food and Drug Administration (FDA) approved Onpattro (Patisiran) developed by Alnylam Pharmaceuticals, Inc. for the treatment of ATTR-PN in adults. Along with this, it holds the commercialization rights to Patisiran in Canada, the U.S., and Western European countries, while Sanofi Genzyme holds the right to the rest of the world.
Targeted therapy is expected to witness the fastest growth during the forecast period owing to increasing initiatives undertaken by key players to support the patients. For instance, Akcea Therapeutics, Inc. operates a patient assistance program “Akcea Connect” to support hATTR amyloidosis patients. Under this program, the eligible person and their families get free, private, and personalized support across the U.S. This will increase awareness about available drug therapies, encouraging general practitioners to prescribe these drugs to transthyretin amyloidosis patients. Hence, the high prescription rate and the high cost of these drugs are major factors fueling the targeted therapy segment growth.
Regional Insights
North America dominated the market with a revenue share of over 65.02% in 2021 and the region is anticipated to maintain its dominance during the forecast period. Increasing novel drugs prescription, proper reimbursement policies, and higher treatment rates in the region are factors supporting transthyretin amyloidosis treatment market growth in the region. The introduction of new therapies in the region is acting as a key market driver. For instance, in July 2019, Health Canada approved ONPATTRO for the treatment of hATTR with polyneuropathy in adults. This approval is projected to have a positive impact on the regional market.
The MEA region is projected to witness the fastest growth over the forecast period. The high unmet treatment needs, increasing prevalence of disease, and rising approval are expected to drive the market in the coming years. Moreover, major factors such as a large target population, good healthcare reforms, and an increase in disease awareness are expected to be the primary growth factors.
Key Players
Market Segmentation
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Transthyretin Amyloidosis Treatment Market
5.1. COVID-19 Landscape: Transthyretin Amyloidosis Treatment Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Transthyretin Amyloidosis Treatment Market, By Type
8.1. Transthyretin Amyloidosis Treatment Market, by Type, 2022-2030
8.1.1. ATTR-PN
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. ATTR-CM
8.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Transthyretin Amyloidosis Treatment Market, By Therapy
9.1. Transthyretin Amyloidosis Treatment Market, by Therapy, 2022-2030
9.1.1. Targeted Therapy
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Supportive Therapy
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Pipeline Therapy
9.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Transthyretin Amyloidosis Treatment Market, By Disease Type
10.1. Transthyretin Amyloidosis Treatment Market, by Disease Type, 2022-2030
10.1.1. Hereditary Transthyretin Amyloidosis
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Wild Type Amyloidosis
10.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Transthyretin Amyloidosis Treatment Market, By Distribution Channel
11.1. Transthyretin Amyloidosis Treatment Market, by Distribution Channel, 2022-2030
11.1.1. Hospital Pharmacies
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Specialty Pharmacies
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Retail Pharmacies
11.1.3.1. Market Revenue and Forecast (2017-2030)
11.1.4. Online Pharmacies
11.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global Transthyretin Amyloidosis Treatment Market, Regional Estimates and Trend Forecast
12.1. North America
12.1.1. Market Revenue and Forecast, by Type (2017-2030)
12.1.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.1.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.1.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.1.5. U.S.
12.1.5.1. Market Revenue and Forecast, by Type (2017-2030)
12.1.5.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.1.5.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.1.5.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.1.6. Rest of North America
12.1.6.1. Market Revenue and Forecast, by Type (2017-2030)
12.1.6.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.1.6.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.1.6.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.2. Europe
12.2.1. Market Revenue and Forecast, by Type (2017-2030)
12.2.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.2.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.2.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.2.5. UK
12.2.5.1. Market Revenue and Forecast, by Type (2017-2030)
12.2.5.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.2.5.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.2.5.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.2.6. Germany
12.2.6.1. Market Revenue and Forecast, by Type (2017-2030)
12.2.6.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.2.6.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.2.6.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.2.7. France
12.2.7.1. Market Revenue and Forecast, by Type (2017-2030)
12.2.7.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.2.7.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.2.7.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.2.8. Rest of Europe
12.2.8.1. Market Revenue and Forecast, by Type (2017-2030)
12.2.8.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.2.8.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.2.8.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.3. APAC
12.3.1. Market Revenue and Forecast, by Type (2017-2030)
12.3.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.3.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.3.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.3.5. India
12.3.5.1. Market Revenue and Forecast, by Type (2017-2030)
12.3.5.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.3.5.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.3.5.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.3.6. China
12.3.6.1. Market Revenue and Forecast, by Type (2017-2030)
12.3.6.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.3.6.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.3.6.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.3.7. Japan
12.3.7.1. Market Revenue and Forecast, by Type (2017-2030)
12.3.7.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.3.7.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.3.7.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.3.8. Rest of APAC
12.3.8.1. Market Revenue and Forecast, by Type (2017-2030)
12.3.8.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.3.8.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.3.8.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.4. MEA
12.4.1. Market Revenue and Forecast, by Type (2017-2030)
12.4.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.4.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.4.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.4.5. GCC
12.4.5.1. Market Revenue and Forecast, by Type (2017-2030)
12.4.5.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.4.5.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.4.5.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.4.6. North Africa
12.4.6.1. Market Revenue and Forecast, by Type (2017-2030)
12.4.6.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.4.6.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.4.6.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.4.7. South Africa
12.4.7.1. Market Revenue and Forecast, by Type (2017-2030)
12.4.7.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.4.7.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.4.7.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.4.8. Rest of MEA
12.4.8.1. Market Revenue and Forecast, by Type (2017-2030)
12.4.8.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.4.8.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.4.8.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.5. Latin America
12.5.1. Market Revenue and Forecast, by Type (2017-2030)
12.5.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.5.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.5.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.5.5. Brazil
12.5.5.1. Market Revenue and Forecast, by Type (2017-2030)
12.5.5.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.5.5.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.5.5.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
12.5.6. Rest of LATAM
12.5.6.1. Market Revenue and Forecast, by Type (2017-2030)
12.5.6.2. Market Revenue and Forecast, by Therapy (2017-2030)
12.5.6.3. Market Revenue and Forecast, by Disease Type (2017-2030)
12.5.6.4. Market Revenue and Forecast, by Distribution Channel (2017-2030)
Chapter 13. Company Profiles
13.1. Pfizer Inc.
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. Johnson & Johnson Services, Inc.
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. Ionis Pharmaceuticals, Inc.
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. Alnylam Pharmaceuticals, Inc.
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. BridgeBio Pharma, Inc.
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. Bristol-Myers Squibb Company
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. Acrotech Biopharma
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. AstraZeneca
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. Astellas Pharma, Inc.
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. Prothena
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others