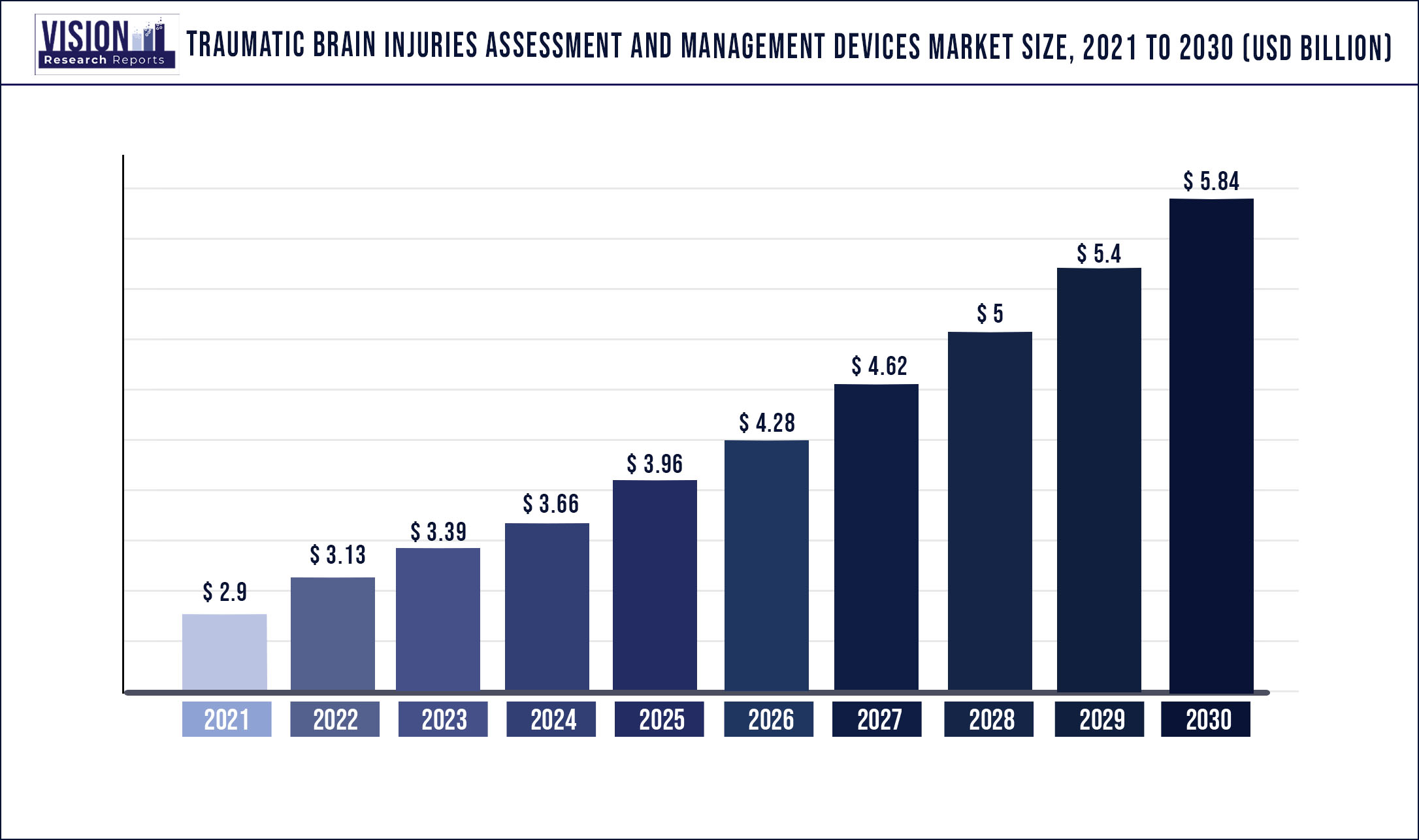
The global traumatic brain injuries assessment and management devices market was valued at USD 2.9 billion in 2021 and it is predicted to surpass around USD 5.84 billion by 2030 with a CAGR of 8.09% from 2022 to 2030.
Report Highlights
Increasing cases of car accidents and injuries caused due to sports, as well as rising patient preference for minimally invasive procedures, are driving the market growth. Falls are one of the leading causes of hospitalization in TBI patients followed by motor vehicle accidents. According to the CDC, presently 3.5 million people are living with permanent TBI-related disability in the U.S.Thus, the increasing incidence of TBIs due to mounting cases of road accidents, and injuries are expected to fuel the product demand.
The COVID-19 outbreak in the year 2020 is expected to have a short-term and moderate impact on the market. Patients suffering from COVID-19 have subsequently shown neurologic symptoms that are expected to create a favorable environment for market growth in the near future. As per the research study published in the NCBI report 2020, certain patients hospitalized for coronavirus infection showed clinical & neurochemical signs of brain injury. This is expected to boost the product demand during the forecast period. The incorporation of the latest technologies and the introduction of new products by market players are also to drive the market during the forecast period. For instance, NanoDx System, launched by BioDirection Company, is a rapid Point-of-Care (POC) test that precisely confirms concussion/TBI in less than two minutes.
Scope of The Report
| Report Coverage | Details |
| Market Size in 2021 | USD 2.9 billion |
| Revenue Forecast by 2030 | USD 5.84 billion |
| Growth rate from 2022 to 2030 | CAGR of 8.09% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segmentation | Device, technique, end-use, region |
| Companies Covered | Integra Lifesciences; BioDirection, Inc.; Nihon Kohden Corp.; Compumedics Ltd.; InfraScan, Inc.; Oculogica; Raumedic AG |
Device Insights
Based on devices, the global market has been further segmented into imaging devices and monitoring devices. The imaging devices segment dominated the global market in 2021 and accounted for the largest share of more than 53.6% of the overall revenue. The imaging devices segment is further sub-categorized into Computed Tomography (CT) scan, Magnetic Resonance Imaging (MRI) devices, X-ray, and others. Due to higher sensitivity, imaging devices are widely used to assess patients with mild or severe TBI. Thus, increasing application scope in the diagnosis and staging of neurological diseases & disorders, particularly TBIs, is expected to drive the segment.
On the other hand, the monitoring devices segment is expected to register the fastest growth rate from 2022 to 2030 on account of the minimally invasive nature of these devices and increasing awareness and improvements in healthcare facilities. In addition, factors, such as greater accuracy of these devices, increasing patient preference for non-invasive procedures, and a high preference among physicians for brain disease progression monitoring, are expected to drive the growth of this segment during the forecast period.
Technique Insights
Based on techniques, the market has been segmented into Intracranial Pressure (ICP) monitoring and partial pressure of oxygen in brain tissue (pBrO2). The ICP monitoring segment dominated the market with a revenue share of more than 54.1% in 2021. This method is considered a gold standard for the measurement of accurate increased intracranial pressure in patients with severe brain injury. Several studies have recommended that raised ICP is the common cause of death in patients with TBI. Therefore, continuous ICP monitoring helps in keeping the pressure levels in a normal range and provides early and effective treatment.
Thus, the advantages offered by ICP monitoring and an increasing number of TBI patients are expected to boost the market growth. However, the partial pressure of oxygen in the brain tissue monitoring segment is expected to grow at the fastest CAGR from 2022 to 2030. Continuous pBrO2 monitoring provides timely information about cerebral oxygen demand in patients suffering from TBI. The pBrO2 monitoring systems provide continuous monitoring of partial pressure of oxygen in brain tissue, thus, providing an early indication of hypoxic events. These factors are anticipated to boost the segment growth during the forecast period.
End-use Insights
Based on end-uses, the global market has been segmented into hospitals, diagnostic centers, and others. The hospital's segment dominated the market in 2021 and accounted for the maximum share of 43.21% of the overall revenue. This growth was mainly attributed to a rise in the number of brain surgeries performed in hospitals as well as an increase in the prevalence of TBI patients across the globe. In addition, hospitals are well equipped with technologically advanced devices that are used by professional neurologists, which is expected to boost the segment growth over the forecast period.
However, the other segment, which includes Ambulatory Surgical Centers (ASCs), research centers, and emergency clinics is expected to register the fastest CAGR from 2022 to 2030. This growth can be attributed to various advantages offered by emergency clinics and ASCs, such as shorter procedure time and same-day discharge, over hospitals. Furthermore, most brain surgeries can now be performed at ASCs, as these procedures turn out to be more advanced and less invasive. Thus, the growth of the other segment is majorly attributed to inexpensive and ongoing advancements in minimally invasive surgical techniques as well as shorter procedure times.
Regional Insights
North America dominated the market with a share of more than 52.1% in 2021. This is attributed to the presence of well-established healthcare facilities in the region, increasing government initiatives, awareness campaigns regarding brain injuries, and greater incidence of TBIs in the continent. For instance, as per the CDC, an expected 2.87 million individuals in the U.S. sustain brain injuries and 1.5 million Americans sustain a TBI annually. In the U.S, at present more than 5.3 million people have a permanent TBI-related disability. These incidence rates involve around 2.5 million TBI-related emergency department visits, 288,000 hospitalizations, and 56,800 deaths.
Thus, contributing to the market growth in the region. The Asia Pacific region is expected to grow at the highest CAGR from 2022 to 2030. This is due to the factors, such as growth in the aging population, a rise in the number of people suffering from TBIs, an increasing incidence of road accidents, and violence. For instance, according to the Ministry of Road Transport and Highways, Government of India, around 4.5 million people were injured in road accidents in 2016. This number has increased by 25.6% from 2007 to 2017. In addition, growing patient affordability has together resulted in a significant number of interventional procedures in the Asia Pacific region.
Key Players
Market Segmentation
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Traumatic Brain Injuries Assessment And Management Devices Market
5.1. COVID-19 Landscape: Traumatic Brain Injuries Assessment And Management Devices Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Traumatic Brain Injuries Assessment And Management Devices Market, By Device
8.1. Traumatic Brain Injuries Assessment And Management Devices Market, by Device, 2022-2030
8.1.1 Imaging Devices
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Magnetic Resonance Imaging (MRI) Devices
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. X-Ray
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Computed Tomography
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Monitoring Devices
8.1.5.1. Market Revenue and Forecast (2017-2030)
8.1.6. Others
8.1.6.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Traumatic Brain Injuries Assessment And Management Devices Market, By Technique
9.1. Traumatic Brain Injuries Assessment And Management Devices Market, by Technique, 2022-2030
9.1.1. Intracranial Pressure (ICP) Monitoring
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Partial Pressure of Oxygen in Brain Tissue (pBrO2)
9.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Traumatic Brain Injuries Assessment And Management Devices Market, By End-use
10.1. Traumatic Brain Injuries Assessment And Management Devices Market, by End-use, 2022-2030
10.1.1. Hospitals
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Diagnostic Centers
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Others
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Traumatic Brain Injuries Assessment And Management Devices Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Device (2017-2030)
11.1.2. Market Revenue and Forecast, by Technique (2017-2030)
11.1.3. Market Revenue and Forecast, by End-use (2017-2030)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Device (2017-2030)
11.1.4.2. Market Revenue and Forecast, by Technique (2017-2030)
11.1.4.3. Market Revenue and Forecast, by End-use (2017-2030)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Device (2017-2030)
11.1.5.2. Market Revenue and Forecast, by Technique (2017-2030)
11.1.5.3. Market Revenue and Forecast, by End-use (2017-2030)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Device (2017-2030)
11.2.2. Market Revenue and Forecast, by Technique (2017-2030)
11.2.3. Market Revenue and Forecast, by End-use (2017-2030)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Device (2017-2030)
11.2.4.2. Market Revenue and Forecast, by Technique (2017-2030)
11.2.4.3. Market Revenue and Forecast, by End-use (2017-2030)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Device (2017-2030)
11.2.5.2. Market Revenue and Forecast, by Technique (2017-2030)
11.2.5.3. Market Revenue and Forecast, by End-use (2017-2030)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Device (2017-2030)
11.2.6.2. Market Revenue and Forecast, by Technique (2017-2030)
11.2.6.3. Market Revenue and Forecast, by End-use (2017-2030)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Device (2017-2030)
11.2.7.2. Market Revenue and Forecast, by Technique (2017-2030)
11.2.7.3. Market Revenue and Forecast, by End-use (2017-2030)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Device (2017-2030)
11.3.2. Market Revenue and Forecast, by Technique (2017-2030)
11.3.3. Market Revenue and Forecast, by End-use (2017-2030)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Device (2017-2030)
11.3.4.2. Market Revenue and Forecast, by Technique (2017-2030)
11.3.4.3. Market Revenue and Forecast, by End-use (2017-2030)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Device (2017-2030)
11.3.5.2. Market Revenue and Forecast, by Technique (2017-2030)
11.3.5.3. Market Revenue and Forecast, by End-use (2017-2030)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Device (2017-2030)
11.3.6.2. Market Revenue and Forecast, by Technique (2017-2030)
11.3.6.3. Market Revenue and Forecast, by End-use (2017-2030)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Device (2017-2030)
11.3.7.2. Market Revenue and Forecast, by Technique (2017-2030)
11.3.7.3. Market Revenue and Forecast, by End-use (2017-2030)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Device (2017-2030)
11.4.2. Market Revenue and Forecast, by Technique (2017-2030)
11.4.3. Market Revenue and Forecast, by End-use (2017-2030)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Device (2017-2030)
11.4.4.2. Market Revenue and Forecast, by Technique (2017-2030)
11.4.4.3. Market Revenue and Forecast, by End-use (2017-2030)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Device (2017-2030)
11.4.5.2. Market Revenue and Forecast, by Technique (2017-2030)
11.4.5.3. Market Revenue and Forecast, by End-use (2017-2030)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Device (2017-2030)
11.4.6.2. Market Revenue and Forecast, by Technique (2017-2030)
11.4.6.3. Market Revenue and Forecast, by End-use (2017-2030)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Device (2017-2030)
11.4.7.2. Market Revenue and Forecast, by Technique (2017-2030)
11.4.7.3. Market Revenue and Forecast, by End-use (2017-2030)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Device (2017-2030)
11.5.2. Market Revenue and Forecast, by Technique (2017-2030)
11.5.3. Market Revenue and Forecast, by End-use (2017-2030)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Device (2017-2030)
11.5.4.2. Market Revenue and Forecast, by Technique (2017-2030)
11.5.4.3. Market Revenue and Forecast, by End-use (2017-2030)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Device (2017-2030)
11.5.5.2. Market Revenue and Forecast, by Technique (2017-2030)
11.5.5.3. Market Revenue and Forecast, by End-use (2017-2030)
Chapter 12. Company Profiles
12.1. Integra Lifesciences
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. BioDirection, Inc.
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Nihon Kohden Corp.
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Compumedics Ltd.
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. InfraScan, Inc.
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Oculogica
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Raumedic AG
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others