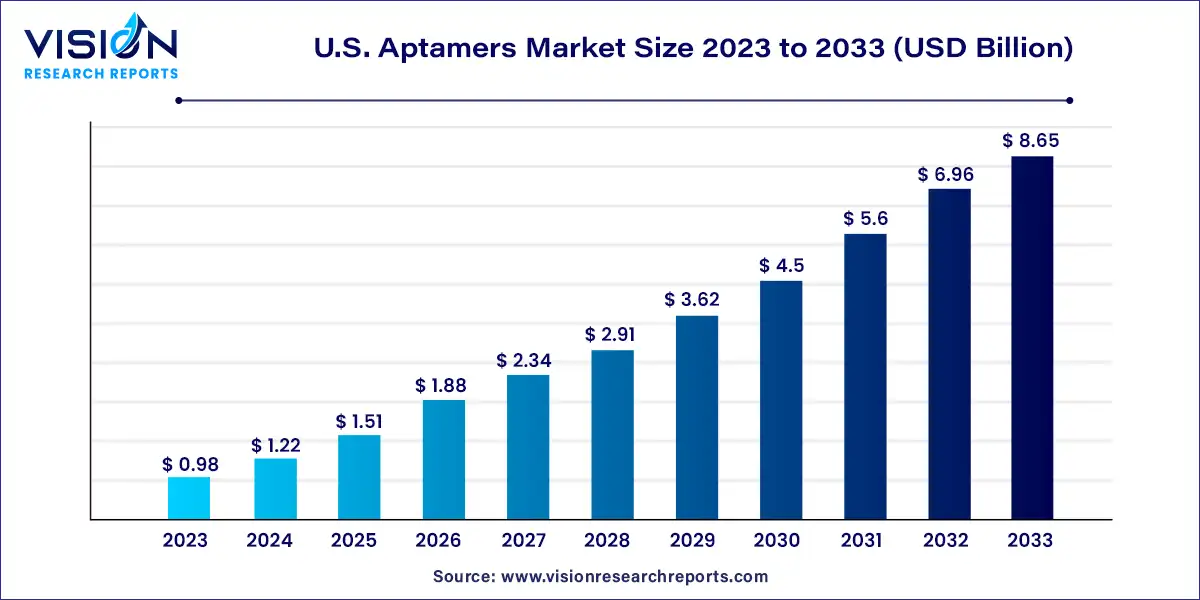
The U.S. aptamers market size was estimated at around USD 0.98 billion in 2023 and it is projected to hit around USD 8.65 billion by 2033, growing at a CAGR of 24.33% from 2024 to 2033.
Aptamers, with their remarkable potential across various applications, have been gaining traction within the United States. This versatile class of molecules, often termed as "chemical antibodies," exhibits high specificity and affinity towards target molecules, ranging from small molecules to proteins and even whole cells. As the biotechnology landscape evolves, aptamers emerge as promising tools for diagnostics, therapeutics, and research applications.
The growth of the U.S. aptamers market is driven by an escalating demand for targeted therapeutics in the healthcare sector is driving the adoption of aptamers due to their precise and customizable nature, which minimizes adverse effects and enhances therapeutic efficacy. Secondly, advancements in SELEX (Systematic Evolution of Ligands by Exponential Enrichment) technology have bolstered the efficient identification and optimization of aptamers against a diverse array of targets, expanding their utility across various industries. Additionally, robust research and development activities, supported by government initiatives and private investments, are fueling innovation in the field, leading to the exploration of novel applications and the broadening of the aptamer market's scope. These factors collectively contribute to the promising growth trajectory of the U.S. aptamers market, positioning it as a key player in the future of diagnostics, therapeutics, and biotechnology within the nation.
In 2023, nucleic acid aptamers dominated the market with a substantial share of 79%, and they are anticipated to experience the fastest growth throughout the forecast period. Currently, numerous companies are investigating the mechanism of action of nucleic acid-based aptamers in the treatment of various disorders, including AMD. In June 2021, IVERIC BIO obtained FDA approval to conduct the GATHER2 phase 3 clinical trial of Zimura under Special Protocol Assessment (SPA). Zimura is specifically designed to address geographic atrophy (GA) secondary to AMD, indicating significant strides in this therapeutic area.
The peptide aptamer segment is poised for lucrative market expansion in the coming years. This growth is attributed to the broad spectrum of applications for peptide aptamers in both diagnostic and therapeutic realms. For example, in August 2021, the Engineering Center for Microtechnology and Diagnostics introduced an advanced biosensor tailored for multiparametric express testing in preclinical diagnostics of cardiovascular diseases. Such innovations underscore the promising trajectory of the peptide aptamer segment, further fueling its market growth.
In 2023, the R&D segment claimed the largest market share at 32%. This dominance is attributed to a multitude of strategic initiatives pursued by key market players, including partnerships, collaborations, and agreements, aimed at advancing the research and development of aptamer-based therapeutic and diagnostic products. A notable example occurred in June 2021, when Ixaka Ltd and SomaLogic formed an R&D collaboration to bolster the discovery and production of aptamer-based bispecific therapeutics. This collaboration focused on assessing the efficacy and safety of antigen-specific SOMAmer reagents.
Expectations are high for the therapeutics segment to experience robust market growth throughout the forecast period. The escalating prevalence of genetic disorders serves as a significant driver for this growth. To optimize cost-effectiveness for therapeutic applications, aptamers are often condensed during synthesis. Furthermore, modifications are made to enhance nuclease resistance and diminish renal filtration rates, thereby augmenting their therapeutic potential.
By Type
By Application
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on U.S. Aptamers Market
5.1. COVID-19 Landscape: U.S. Aptamers Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. U.S. Aptamers Market, By Type
8.1. U.S. Aptamers Market, by Type, 2024-2033
8.1.1. Nucleic Acid Aptamer
8.1.1.1. Market Revenue and Forecast (2021-2033)
8.1.2. Peptide Aptamer
8.1.2.1. Market Revenue and Forecast (2021-2033)
Chapter 9. U.S. Aptamers Market, By Application
9.1. U.S. Aptamers Market, by Application, 2024-2033
9.1.1. Diagnostics
9.1.1.1. Market Revenue and Forecast (2021-2033)
9.1.2. Therapeutics
9.1.2.1. Market Revenue and Forecast (2021-2033)
9.1.3. Research and Development
9.1.3.1. Market Revenue and Forecast (2021-2033)
9.1.4. Others
9.1.4.1. Market Revenue and Forecast (2021-2033)
Chapter 10. U.S. Aptamers Market, Regional Estimates and Trend Forecast
10.1. U.S.
10.1.1. Market Revenue and Forecast, by Type (2021-2033)
10.1.2. Market Revenue and Forecast, by Application (2021-2033)
Chapter 11. Company Profiles
11.1. SomaLogic
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Aptamer Group
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Aptadel Therapeutics
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. Base Pair Biotechnologies
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. LTE Scientific
11.5. Noxxon Pharma
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Vivonics Inc.
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Aptagen, LLC
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. TriLink Biotechnologies
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Altermune LLC
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. AM Biotechnologies
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others