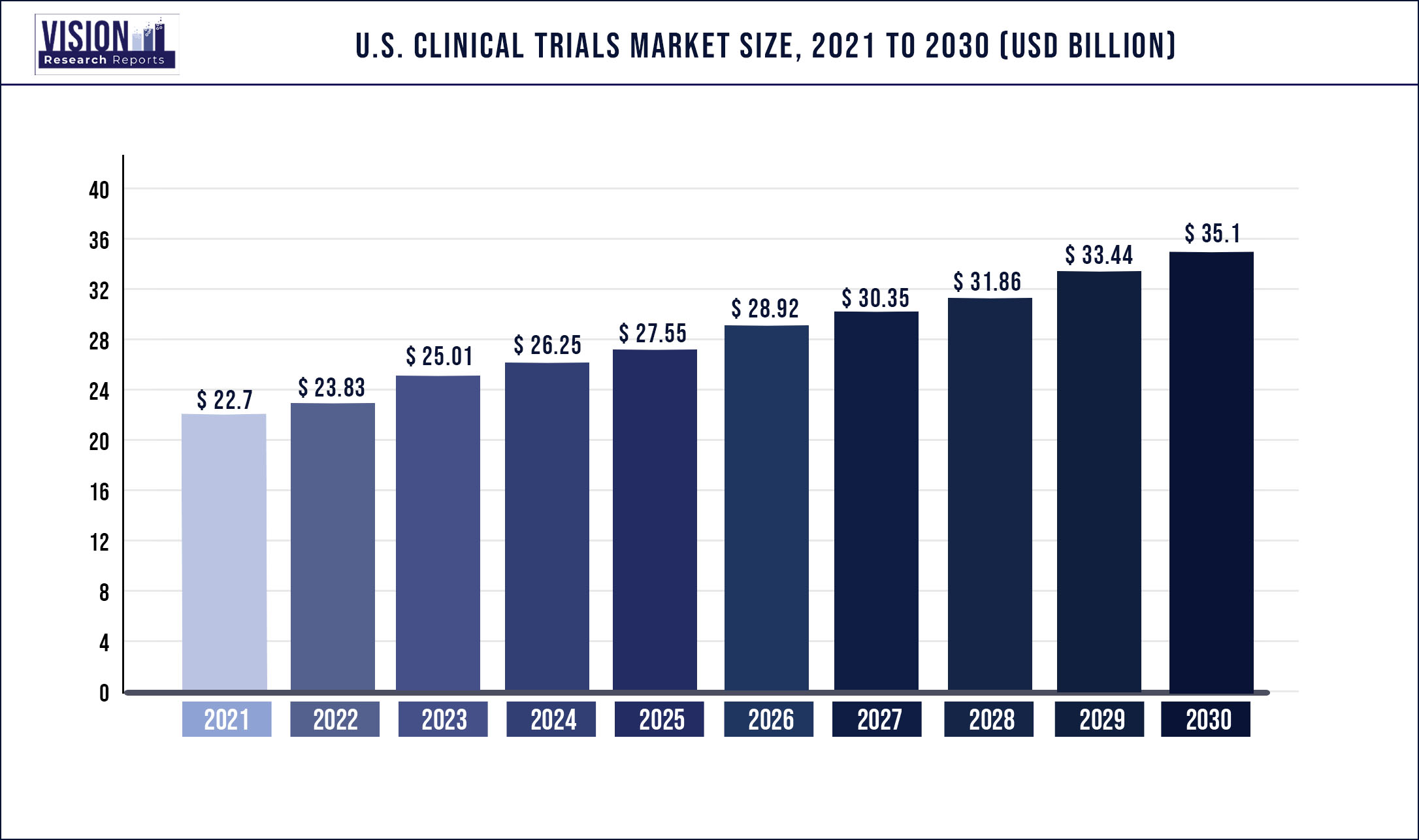
The U.S. clinical trials market size was estimated at USD 22.7 billion in 2021 and is expected to surpass USD 35.1 billion by 2030 and expanding growth at a CAGR of 4.96% from 2022 to 2030.
The shift towards personalized medicine, increasing R&D investments, and growing disease variation, as well as prevalence, are favoring the market growth. There has been a rise in the prevalence of diseases due to which, pharmaceutical companies are increasing their R&D expenditure to launch new therapeutics in the market. That has, in turn, increased the clinical trial value and complexity. Furthermore, the lower cost of clinical trials as a result of outsourcing services has strengthened clinical trial services in the U.S. As the number of people affected by the coronavirus has reached one million, the rapid spread of the virus around the globe is causing significant challenges to clinical trials and may forever change the way future trials are conducted.
The hospitals & clinics are less accessible, and it is unsafe and extremely difficult for patients enrolled in clinical trials to travel to sites. The current pandemic has placed a huge strain on the industry and is also likely to affect data analysis and interpretation. But that has also led to discoveries of conducting clinical trials effectively and efficiently. One such approach is to use Artificial Intelligence (AI) technology for clinical trials. Artificial intelligence uses software apps, data analytics, virtual monitoring, and online platforms to conduct every step of the clinical trial process, including patient recruitment, counseling, and consent. It helps in increasing precision and focuses on the efficiency of clinical trials. Thus, AI-generated technology has been gaining momentum as a healthcare offering, and the COVID-19 is adding more fuel to this initiative.
Scoep of the Report
| Report Coverage | Details |
| Market Size in 2021 | USD 22.7 Billion |
| Revenue Forecast by 2030 | USD 35.1 Billion |
| Growth Rate from 2022 to 2030 | CAGR of 4.96% |
| Base Year | 2021 |
| Forecast Data | 2022 to 2030 |
Phase Insights
The market was led by the phase III segment, which accounted for the largest revenue share of more than 54% in 2021. This is largely due to the fact that the phase III clinical trials are most crucial as they involve 300 to 3000 participants and have a longer treatment period. Thus, it is the most expensive phase.
The second most expensive phase is phase II. It accounted for a significant revenue share in 2021 and the segment is estimated to expand at the fastest CAGR from 2022 to 2030. It plays a very important role in the efficacy study, and also finalizing the dose is done in this phase. Phase II trials had the highest number of projects in 2016 and this trend is expected to continue owing to increasing investments in R&D by industry and non-industry sponsors.
Study Design Insights
The interventional design segment dominated the market in 2021 and accounted for a revenue share of over 84%. The segment is expected to register the second-fastest CAGR during the forecasted period. Interventional methods are used to estimate the direct impact of the treatment and preventive measures that can be taken to treat a disease. Every trial has a limitation that needs to be figured out at the initial stages to minimize it. Interventional designs help identify the weakness and strengths of a clinical trial.
Expanded access trials are projected to grow at a CAGR of 4.4% during the forecasted period. It will be a prospective approach for patients having a serious disease or condition, which is life-threatening. In this, a patient is allowed to carry out treatment outside of a clinical trial when no satisfactory therapies are available. The main driver of this segment is increasing innovation. For instance, HUTCHMED, in collaboration with surufatinib, has started an Expanded Access Program (EAP) in the U.S. for Neuroendocrine Tumors for patients having life-threatening conditions that can’t be treated with available medications or clinical trials.
Indication Insights
The oncology segment accounted for the largest market share of over 37% in 2021 and is expected to maintain its position growing at the fastest CAGR over the forecasted period. This growth is due to the growing number of cases of cancer. Thus, a large amount of expenditure is spent on oncology clinical trials.
According to the reports by the U.S. FDA and various other sources, USD 38.0 billion and more is spent by pharmaceutical companies on the development of medications for various cancer diseases. The cardiovascular segment is anticipated to grow at a CAGR of 5.5% over the forecasted period. This is due to the fact that over 18 million people in the U.S. have Cardiovascular Diseases (CVDs), and around a quarter of them die due to incomplete care. This increases the demand for cost-efficient drugs and has been a major driver for this segment.
Key Players
Market Segmentation
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others