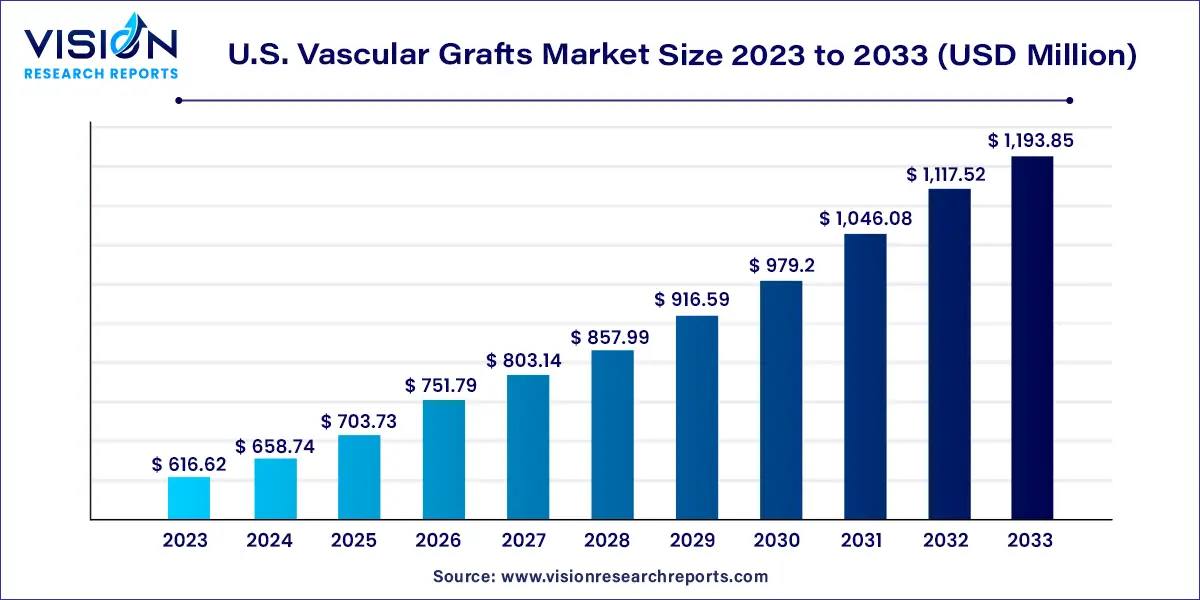
The U.S. vascular grafts market size was estimated at around USD 616.62 million in 2023 and it is projected to hit around USD 1,193.85 million by 2033, growing at a CAGR of 6.83% from 2024 to 2033. The U.S. vascular grafts market is driven by the rising incidence of cardiovascular diseases in the u.s. population, pediatric congenital heart surgeries, development of novel prosthetic grafts, patient outcomes and minimally invasive procedures, and treatment of unruptured saccular aneurysms.
The U.S. vascular grafts market is a crucial component of the country's healthcare landscape, contributing significantly to the field of vascular surgery and patient care. This overview aims to provide a comprehensive understanding of the current state, trends, and factors influencing the U.S. vascular grafts market.
The growth of the U.S. Vascular Grafts market is influenced by several key factors. Technological advancements play a pivotal role, as ongoing innovations in graft materials and design contribute to enhanced performance and durability, thereby driving increased demand. Additionally, the rising prevalence of vascular diseases in the U.S. population, coupled with the aging demographic, serves as a significant growth driver. The expanding incidence of cardiovascular diseases and peripheral vascular disorders underscores the crucial role vascular grafts play in addressing these health challenges. Despite regulatory complexities and the high cost associated with advanced grafts presenting challenges, they also create opportunities for market players to innovate, expand their market reach, and ultimately improve patient outcomes. As a result, the U.S. vascular grafts market is poised for continued growth, fueled by a combination of technological advancements and the evolving healthcare needs of the population.
In 2023, the cardiac aneurysm segment took the lead in the vascular grafts market, commanding a substantial 52% of the revenue share. This dominance is attributed to the rising incidence of cardiovascular diseases and advancements in developing sophisticated tissue-engineered grafts for pediatric congenital heart surgeries. The market is poised for further growth, driven by factors like the introduction of innovative prosthetic grafts boasting enhanced porosity and efficiency, coupled with an increasing acceptance of these grafts. Additionally, the prevalence of unruptured saccular aneurysms impacting intracranial artery flow is expected to contribute to the expanding market for vascular grafts throughout the forecast period.
Notably, the vascular occlusion segment is anticipated to experience the highest growth in the upcoming forecast period. The surge is fueled by the escalating prevalence of vascular occlusive diseases and the convenient accessibility of graft procedures, particularly involving the saphenous vein graft. Technological progressions leading to the emergence of novel therapies, such as warfarin, designed to mitigate the severity of acute ischemia following PTFE graft occlusion, are expected to mitigate risks associated with prosthetic vascular grafts. Consequently, this development is projected to bolster the adoption of vascular grafts over the forecast period.
In 2023, the polytetrafluoroethylene (PTFE) vascular grafts segment emerged as the leader in the vascular grafts market, commanding a substantial 46% of the revenue share. This dominance is primarily driven by the escalating demand for cutting-edge engineered prosthetics and technologically advanced products. The noteworthy factors contributing to the largest share in this segment include the heightened demand for novel engineered prosthetics and technologically advanced products. Additionally, the low risk of degradation and infection associated with polytetrafluoroethylene grafts significantly contributes to their market dominance. PTFE, designed with advanced technology, exhibits characteristics such as minimal blood loss, high delamination resistance, and applicability in extra-anatomical procedures and peripheral bypasses. Consequently, PTFE vascular grafts are poised to gain more market share throughout the forecast period.
Concurrently, the polyester vascular grafts segment is anticipated to witness substantial growth in the forecast period. This growth can be attributed to factors such as the easy availability of raw materials, high tensile strength, and remarkable durability of polyester grafts. Moreover, the widespread use of Dacron durable polyester, infused with mitogenic properties, and the increasing number of clinical trials aimed at developing highly durable polyester grafts are expected to propel market growth over the forecast period.
In 2023, the endovascular stent grafts segment emerged as the frontrunner in the vascular grafts market, commanding a significant 65% of the revenue share. This leadership is a consequence of the escalating number of abdominal aortic aneurysm procedures, coupled with a reduced potential for access-site complications. Additionally, the lower mortality rate associated with endovascular stent graft treatment and ongoing technological advancements in novel product development are key factors expected to positively impact market growth throughout the forecast period. Moreover, these stent-grafts, when combined with antibiotics, present an alternative treatment for mycotic aneurysms. Given the prolonged duration and excessive blood loss associated with open surgeries, the demand for minimally invasive endovascular stent grafts is anticipated to gain substantial momentum in the coming forecast period.
Concurrently, the peripheral vascular grafts segment is poised to experience significant growth in the forecast period. This growth is primarily driven by the diverse range of medical applications and advancements in intraoperative techniques. The increasing prevalence of Peripheral Artery Diseases (PADs) stands out as a key factor contributing to market expansion. Anticipated technological advancements in developing novel products for PAD treatment are expected to fuel demand for peripheral vascular grafts in the forecast period.
By Product
By Application
By Raw Material
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Application Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on U.S. Vascular Grafts Market
5.1. COVID-19 Landscape: U.S. Vascular Grafts Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. U.S. Vascular Grafts Market, By Application
8.1. U.S. Vascular Grafts Market, by Application, 2024-2033
8.1.1 Hemodialysis Access Grafts
8.1.1.1. Market Revenue and Forecast (2021-2033)
8.1.2. Endovascular Stent Grafts
8.1.2.1. Market Revenue and Forecast (2021-2033)
8.1.3. Coronary Artery By-Pass Grafts
8.1.3.1. Market Revenue and Forecast (2021-2033)
8.1.4. Vascular Grafts for Aorta Disease
8.1.4.1. Market Revenue and Forecast (2021-2033)
8.1.5. Peripheral Vascular Grafts
8.1.5.1. Market Revenue and Forecast (2021-2033)
Chapter 9. U.S. Vascular Grafts Market, By Application
9.1. U.S. Vascular Grafts Market, by Application, 2024-2033
9.1.1. Cardiac Aneurysm
9.1.1.1. Market Revenue and Forecast (2021-2033)
9.1.2. Kidney Failure
9.1.2.1. Market Revenue and Forecast (2021-2033)
9.1.3. Vascular Occlusion
9.1.3.1. Market Revenue and Forecast (2021-2033)
9.1.4. Coronary Artery Disease
9.1.4.1. Market Revenue and Forecast (2021-2033)
Chapter 10. U.S. Vascular Grafts Market, By Raw Material
10.1. U.S. Vascular Grafts Market, by Raw Material, 2024-2033
10.1.1. Synthetic Vascular Grafts
10.1.1.1. Market Revenue and Forecast (2021-2033)
10.1.2. Biological Vascular Grafts
10.1.2.1. Market Revenue and Forecast (2021-2033)
10.1.3. Hybrid Vascular Grafts
10.1.3.1. Market Revenue and Forecast (2021-2033)
Chapter 11. U.S. Vascular Grafts Market, Regional Estimates and Trend Forecast
11.1. U.S.
11.1.1. Market Revenue and Forecast, by Application (2021-2033)
11.1.2. Market Revenue and Forecast, by Application (2021-2033)
11.1.3. Market Revenue and Forecast, by Raw Material (2021-2033)
Chapter 12. Company Profiles
12.1. Medtronic.
12.1.1. Company Overview
12.1.2. Application Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Terumo Corporation.
12.2.1. Company Overview
12.2.2. Application Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. LeMaitre Vascular, Inc.
12.3.1. Company Overview
12.3.2. Application Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Getinge AB.
12.4.1. Company Overview
12.4.2. Application Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. BD (Becton Dickinson).
12.5.1. Company Overview
12.5.2. Application Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Abbott
12.6.1. Company Overview
12.6.2. Application Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. B. Braun Melsungen AG.
12.7.1. Company Overview
12.7.2. Application Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Abbott
12.8.1. Company Overview
12.8.2. Application Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. W. L. Gore and Associates, Inc.
12.9.1. Company Overview
12.9.2. Application Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. MicroPort Scientific Corporation
12.10.1. Company Overview
12.10.2. Application Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others