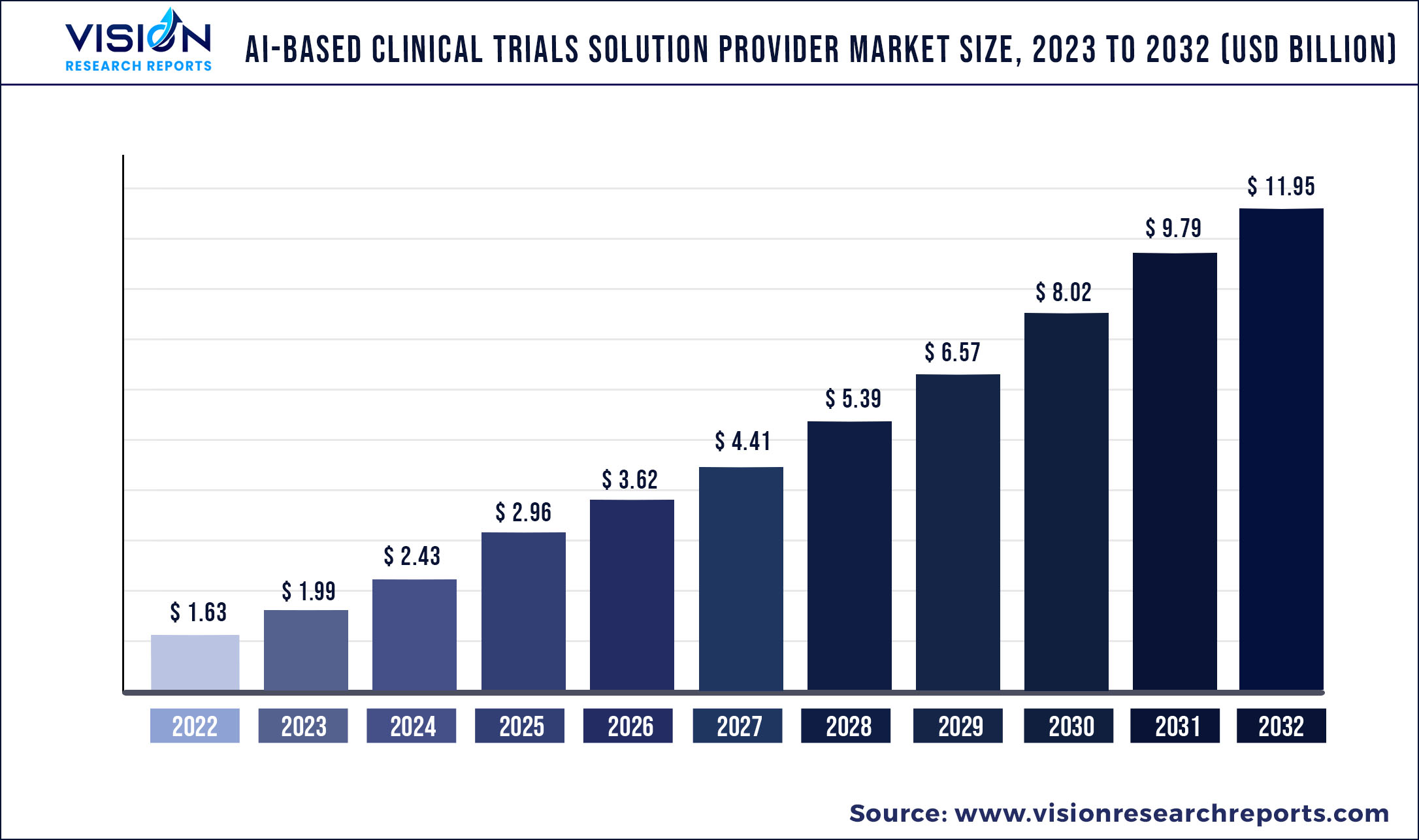
The global AI-based clinical trials solution provider market size was estimated at around USD 1.63 billion in 2022 and it is projected to hit around USD 11.95 billion by 2032, growing at a CAGR of 22.04% from 2023 to 2032.
Key Pointers
Report Scope of the AI-based Clinical Trials Solution Provider Market
| Report Coverage | Details |
| Market Size in 2022 | USD 1.63 billion |
| Revenue Forecast by 2032 | USD 11.95 billion |
| Growth rate from 2023 to 2032 | CAGR of 22.04% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Covered | Unlearn.AI, Inc.; Saama Technologies; Antidote Technologies, Inc.; Phesi; Deep 6 AI; Innoplexus; Mendel.ai; Intelligencia; Median Technologies; Symphony AI; BioAge Labs, Inc.; AiCure, LLC; CONSILX; DEEP LENS AI; Halo Health Systems; Pharmaseal; Ardigen; Trials.Ai; Koneksa Health; Euretos; BioSymetrics; Google- Verily; GNS Healthcare; IBM Watson; Exscientia |
The increasing adoption of AI-based platforms to improve the productivity and efficacy of trials at various stages is driving the market for AI-based clinical trial solution providers. Also, the supportive initiatives by the private and public sectors for different therapeutic areas are some of the factors propelling the market growth. Furthermore, the rising awareness and diversified applications provided by AI in the field of clinical trials such as designing drug trials, improved patient selection, site selection, patient monitoring, etc. is bolstering the market growth.
The utilization of AI in drug trials can be useful to improve the cost, clinical outcomes, and time required for drug trials, as drug development is a cost-intensive and time-consuming process. For instance, in January 2020, Recursion Pharma and Takeda entered into a research collaboration for rare diseases, which resulted in the evaluation of preclinical and clinical molecules in over 60 unique indications in less than 18 months. Moreover, AI can also be used to reduce the bias in medical data. For instance, Genentech collaborated with Stanford University to use an open-source AI system to fight and reduce bias in drug trials. In addition, due to the shifting trend from traditional practices to technology-based approaches, major pharmaceutical companies are highly implementing AI-based technologies in clinical trials, thereby boosting the market growth.
Furthermore, the rising penetration of AI in drug trials and the availability of AI-based solutions can help in different aspects of clinical trials such as drug trial design, patient enrichment & enrollment, investigator and site selection, patient monitoring, medication adherence, and many more, which is boosting the growth of the market for AI-based clinical trial solution providers. Patient eligibility and enrollment are some of the important steps for the success of the overall drug trial and as per research, 85% of the drug trials are delayed during patient recruitment, and 30% are terminated early due to recruitment failure. AI-based platforms are proving to be beneficial in reducing this hurdle. Hence, many researchers are using AI for the drug trial process, thereby fostering the growth of the market for AI-based clinical trial solution providers.
In addition, the COVID-19 outbreak led to the rise in the utilization of AI-based technologies. Increasing adoption of technologically advanced solutions for drug discovery and development and recruited patient data analysis are some of the factors responsible for the increase in penetration of AI-based drug development and drug trial solutions. The pharmaceutical companies, CROs, and academia shifted their focus from the traditional drug development process to the AI-based solution to improve the clinical outcomes and for the minimization of cost and time required for the drug trials. Also, the decentralized drug trials witnessed a boost as many trials were on hold due to COVID-19, due to which many key players turned toward the collection of patient data available.
AI-based Clinical Trials Solution Provider Market Segmentations:
| By Clinical Trial Phase | By Therapeutic Application | By End-user |
|
Phase-I Phase-II Phase-III |
Oncology Cardiovascular Diseases Neurological Diseases or Conditions Metabolic Diseases Infectious Diseases Others |
Pharmaceutical Companies Academia Others |
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Clinical Trial Phase Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on AI-based Clinical Trials Solution Provider Market
5.1. COVID-19 Landscape: AI-based Clinical Trials Solution Provider Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global AI-based Clinical Trials Solution Provider Market, By Clinical Trial Phase
8.1. AI-based Clinical Trials Solution Provider Market, by Clinical Trial Phase, 2023-2032
8.1.1 Phase-I
8.1.1.1. Market Revenue and Forecast (2020-2032)
8.1.2. Phase-II
8.1.2.1. Market Revenue and Forecast (2020-2032)
8.1.3. Phase-III
8.1.3.1. Market Revenue and Forecast (2020-2032)
Chapter 9. Global AI-based Clinical Trials Solution Provider Market, By Therapeutic Application
9.1. AI-based Clinical Trials Solution Provider Market, by Therapeutic Application, 2023-2032
9.1.1. Oncology
9.1.1.1. Market Revenue and Forecast (2020-2032)
9.1.2. Cardiovascular Diseases
9.1.2.1. Market Revenue and Forecast (2020-2032)
9.1.3. Neurological Diseases or Conditions
9.1.3.1. Market Revenue and Forecast (2020-2032)
9.1.4. Metabolic Diseases
9.1.4.1. Market Revenue and Forecast (2020-2032)
9.1.5. Infectious Diseases
9.1.5.1. Market Revenue and Forecast (2020-2032)
9.1.6. Others
9.1.6.1. Market Revenue and Forecast (2020-2032)
Chapter 10. Global AI-based Clinical Trials Solution Provider Market, By End-user
10.1. AI-based Clinical Trials Solution Provider Market, by End-user, 2023-2032
10.1.1. Pharmaceutical Companies
10.1.1.1. Market Revenue and Forecast (2020-2032)
10.1.2. Academia
10.1.2.1. Market Revenue and Forecast (2020-2032)
10.1.3. Others
10.1.3.1. Market Revenue and Forecast (2020-2032)
Chapter 11. Global AI-based Clinical Trials Solution Provider Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.1.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.1.3. Market Revenue and Forecast, by End-user (2020-2032)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.1.4.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.1.4.3. Market Revenue and Forecast, by End-user (2020-2032)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.1.5.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.1.5.3. Market Revenue and Forecast, by End-user (2020-2032)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.2.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.2.3. Market Revenue and Forecast, by End-user (2020-2032)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.2.4.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.2.4.3. Market Revenue and Forecast, by End-user (2020-2032)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.2.5.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.2.5.3. Market Revenue and Forecast, by End-user (2020-2032)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.2.6.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.2.6.3. Market Revenue and Forecast, by End-user (2020-2032)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.2.7.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.2.7.3. Market Revenue and Forecast, by End-user (2020-2032)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.3.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.3.3. Market Revenue and Forecast, by End-user (2020-2032)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.3.4.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.3.4.3. Market Revenue and Forecast, by End-user (2020-2032)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.3.5.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.3.5.3. Market Revenue and Forecast, by End-user (2020-2032)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.3.6.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.3.6.3. Market Revenue and Forecast, by End-user (2020-2032)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.3.7.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.3.7.3. Market Revenue and Forecast, by End-user (2020-2032)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.4.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.4.3. Market Revenue and Forecast, by End-user (2020-2032)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.4.4.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.4.4.3. Market Revenue and Forecast, by End-user (2020-2032)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.4.5.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.4.5.3. Market Revenue and Forecast, by End-user (2020-2032)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.4.6.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.4.6.3. Market Revenue and Forecast, by End-user (2020-2032)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.4.7.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.4.7.3. Market Revenue and Forecast, by End-user (2020-2032)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.5.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.5.3. Market Revenue and Forecast, by End-user (2020-2032)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.5.4.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.5.4.3. Market Revenue and Forecast, by End-user (2020-2032)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Clinical Trial Phase (2020-2032)
11.5.5.2. Market Revenue and Forecast, by Therapeutic Application (2020-2032)
11.5.5.3. Market Revenue and Forecast, by End-user (2020-2032)
Chapter 12. Company Profiles
12.1. Unlearn.AI, Inc.
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Saama Technologies
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Antidote Technologies, Inc.
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Phesi
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Deep 6 AI
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Innoplexus
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Mendel.ai
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Intelligencia
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Median Technologies
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Symphony AI
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others