The global biological safety testing products and services market size was estimated at around USD 4.58 billion in 2023 and it is projected to hit around USD 12.7 billion by 2033, growing at a CAGR of 10.74% from 2024 to 2033. The biological safety testing products and services market plays a pivotal role in ensuring the safety, quality, and regulatory compliance of a wide array of healthcare products. This sector encompasses a diverse range of testing solutions specifically designed for pharmaceuticals, biologics, medical devices, and other related products. This overview provides a snapshot of the key components, market dynamics, and factors influencing the landscape of Biological Safety Testing.
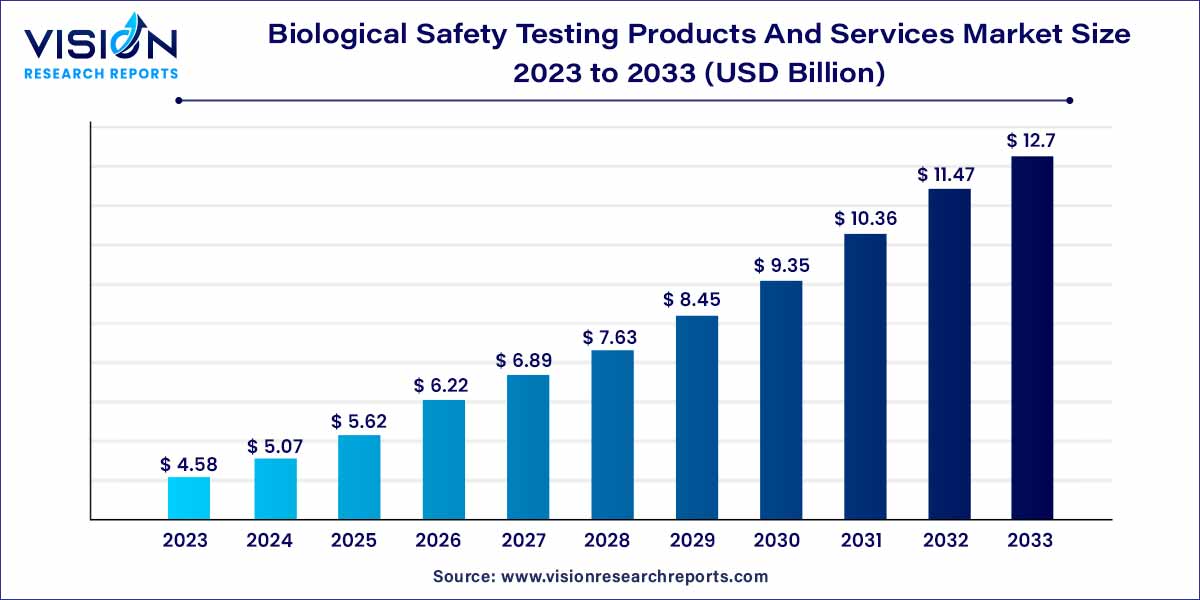
The growth of the biological safety testing products and services market is propelled by several key factors. Firstly, the increasing stringency of regulatory frameworks, particularly from agencies such as the FDA and EMA, necessitates rigorous safety testing, driving the demand for advanced testing products and services. Secondly, advancements in testing technologies, including next-generation sequencing and cell-based assays, contribute to the market's growth by enhancing the accuracy and efficiency of safety assessments. Thirdly, the rising prevalence of infectious diseases and global health challenges accentuates the importance of reliable and efficient biological safety testing in pharmaceutical and biopharmaceutical development. Moreover, the industry's response to these challenges through research and development efforts fosters innovation, creating new opportunities for market expansion. Overall, the convergence of regulatory compliance, technological progress, and the imperative for heightened safety standards collectively underpin the growth trajectory of the biological safety testing products and services market.
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 10.74% |
| Market Revenue by 2033 | USD 12.7 billion |
| Revenue Share of North America in 2023 | 36% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units)Companies Covered |
The reagents and kits accounted for the largest market share of 41% in 2023. Reagents & Kits and Instruments. The domain of Reagents & Kits encompasses an array of essential tools designed to facilitate precise and thorough safety assessments in various healthcare product developments. These reagents and kits play a pivotal role in microbial testing, endotoxin testing, bioburden testing, and other crucial aspects of safety evaluation. Their significance lies in their ability to provide accurate results, ensuring the absence of harmful microorganisms and contaminants in pharmaceuticals, biologics, and medical devices.
The instruments segment is expected to grow at fastest growth rate during the forecast period. These instruments are designed to streamline and optimize testing methodologies, incorporating advanced technologies such as next-generation sequencing and rapid microbial detection methods. The instruments contribute to the efficiency and reliability of safety assessments, catering to the evolving needs of the pharmaceutical and biopharmaceutical industries. As the demand for sophisticated testing solutions continues to rise, the market for biological safety testing instruments remains at the forefront of technological innovation, providing essential tools for accurate and timely safety evaluations.
The vaccine and therapeutics segment held the largest share of 24% in 2023. Within this domain, the emphasis lies on ensuring the safety and efficacy of vaccines and therapeutic agents. Rigorous safety testing becomes imperative to verify the absence of microbial contaminants, endotoxins, and other potential risks in these products. As the demand for vaccines and therapeutic innovations continues to grow globally, the Biological Safety Testing Products and Services play an instrumental role in upholding the integrity of these crucial healthcare interventions.
The gene therapy segment is predicted to grow at the fastest CAGR during the forecast period. The market application extends to the burgeoning field of Gene Therapy. With advancements in genetic medicine gaining momentum, the safety assessment of gene therapy products becomes paramount. Biological Safety Testing facilitates the identification and mitigation of risks associated with gene therapy, ensuring the delivery of safe and effective treatments. The complexities inherent in gene therapy formulations necessitate specialized testing protocols, making biological safety testing a cornerstone in the development of gene-based therapeutic solutions. As the field of gene therapy expands, the market for Biological Safety Testing Products and Services assumes a pivotal role in supporting the safety and success of these groundbreaking medical advancements.
Endotoxin tests dominated the global market with the largest market share of 22% in 2023. Endotoxin testing stands as a critical pillar in ensuring the safety of pharmaceuticals, biologics, and medical devices by detecting the presence of bacterial endotoxins. These tests are indispensable in verifying that healthcare products are free from potentially harmful components, thereby upholding the stringent regulatory standards set forth by health authorities. The precision and reliability of Endotoxin Tests contribute significantly to the overall safety assurance in the development and production of a wide array of healthcare interventions.
The bioburden tests segment is expected to grow at fastest growth rate over the forecast period. Bioburden Tests play a crucial role in assessing the microbial load in raw materials and finished products. These tests provide insights into the quantity and types of microorganisms present, aiding in the identification of potential contamination risks. By establishing microbial control measures, Bioburden Tests contribute to the overall safety and quality of pharmaceuticals and medical devices. The meticulous evaluation of bioburden ensures that healthcare products meet the necessary standards, aligning with the industry's commitment to delivering safe and effective interventions to patients worldwide.
North America region dominated the market with the largest market share of 36% in 2023. In North America, a prominent market presence is driven by a robust healthcare infrastructure and stringent regulatory standards. The region, led by the United States, is a focal point for advanced technological innovations and a stronghold for industry leaders committed to delivering cutting-edge safety testing solutions.
The Asia Pacific market is anticipated to grow at a significant CAGR over the forecast period. The Asia-Pacific region emerges as a burgeoning market, driven by the rapid expansion of the pharmaceutical and biotechnology sectors. With increasing investments in healthcare infrastructure, particularly in countries like China and India, the demand for Biological Safety Testing Products and Services is on the rise. This region represents a significant growth opportunity for market players, as it becomes a focal point for research and development activities and manufacturing operations.
By Product
By Application
By Test Type
By Region
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Product Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Biological Safety Testing Products And Services Market
5.1. COVID-19 Landscape: Biological Safety Testing Products And Services Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Biological Safety Testing Products And Services Market, By Product
8.1. Biological Safety Testing Products And Services Market, by Product, 2024-2033
8.1.1 Reagents & Kits
8.1.1.1. Market Revenue and Forecast (2021-2033)
8.1.2. Instruments
8.1.2.1. Market Revenue and Forecast (2021-2033)
8.1.3. Services
8.1.3.1. Market Revenue and Forecast (2021-2033)
Chapter 9. Global Biological Safety Testing Products And Services Market, By Application
9.1. Biological Safety Testing Products And Services Market, by Application, 2024-2033
9.1.1. Vaccines & Therapeutics
9.1.1.1. Market Revenue and Forecast (2021-2033)
9.1.2. Vaccines
9.1.2.1. Market Revenue and Forecast (2021-2033)
9.1.3. Monoclonal Antibodies
9.1.3.1. Market Revenue and Forecast (2021-2033)
9.1.4. Recombinant Protein
9.1.4.1. Market Revenue and Forecast (2021-2033)
9.1.5. Blood & Blood-based Products
9.1.5.1. Market Revenue and Forecast (2021-2033)
9.1.6. Gene Therapy
9.1.6.1. Market Revenue and Forecast (2021-2033)
9.1.7. Tissue & Tissue-based Products
9.1.7.1. Market Revenue and Forecast (2021-2033)
9.1.8. Stem Cell
9.1.8.1. Market Revenue and Forecast (2021-2033)
Chapter 10. Global Biological Safety Testing Products And Services Market, By Test Type
10.1. Biological Safety Testing Products And Services Market, by Test Type, 2024-2033
10.1.1. Endotoxin Tests
10.1.1.1. Market Revenue and Forecast (2021-2033)
10.1.2. Sterility Tests
10.1.2.1. Market Revenue and Forecast (2021-2033)
10.1.3. Cell Line Authentication & Characterization Tests
10.1.3.1. Market Revenue and Forecast (2021-2033)
10.1.4. Bioburden Tests
10.1.4.1. Market Revenue and Forecast (2021-2033)
10.1.5. Adventitious Agent Detection Tests
10.1.5.1. Market Revenue and Forecast (2021-2033)
10.1.6. Residual Host Contamination Detection Tests
10.1.7.1. Market Revenue and Forecast (2021-2033)
10.1.7. Others
10.1.6.1. Market Revenue and Forecast (2021-2033)
Chapter 11. Global Biological Safety Testing Products And Services Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Product (2021-2033)
11.1.2. Market Revenue and Forecast, by Application (2021-2033)
11.1.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Product (2021-2033)
11.1.4.2. Market Revenue and Forecast, by Application (2021-2033)
11.1.4.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Product (2021-2033)
11.1.5.2. Market Revenue and Forecast, by Application (2021-2033)
11.1.5.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Product (2021-2033)
11.2.2. Market Revenue and Forecast, by Application (2021-2033)
11.2.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Product (2021-2033)
11.2.4.2. Market Revenue and Forecast, by Application (2021-2033)
11.2.4.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Product (2021-2033)
11.2.5.2. Market Revenue and Forecast, by Application (2021-2033)
11.2.5.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Product (2021-2033)
11.2.6.2. Market Revenue and Forecast, by Application (2021-2033)
11.2.6.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Product (2021-2033)
11.2.7.2. Market Revenue and Forecast, by Application (2021-2033)
11.2.7.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Product (2021-2033)
11.3.2. Market Revenue and Forecast, by Application (2021-2033)
11.3.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Product (2021-2033)
11.3.4.2. Market Revenue and Forecast, by Application (2021-2033)
11.3.4.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Product (2021-2033)
11.3.5.2. Market Revenue and Forecast, by Application (2021-2033)
11.3.5.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Product (2021-2033)
11.3.6.2. Market Revenue and Forecast, by Application (2021-2033)
11.3.6.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Product (2021-2033)
11.3.7.2. Market Revenue and Forecast, by Application (2021-2033)
11.3.7.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Product (2021-2033)
11.4.2. Market Revenue and Forecast, by Application (2021-2033)
11.4.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Product (2021-2033)
11.4.4.2. Market Revenue and Forecast, by Application (2021-2033)
11.4.4.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Product (2021-2033)
11.4.5.2. Market Revenue and Forecast, by Application (2021-2033)
11.4.5.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Product (2021-2033)
11.4.6.2. Market Revenue and Forecast, by Application (2021-2033)
11.4.6.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Product (2021-2033)
11.4.7.2. Market Revenue and Forecast, by Application (2021-2033)
11.4.7.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Product (2021-2033)
11.5.2. Market Revenue and Forecast, by Application (2021-2033)
11.5.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Product (2021-2033)
11.5.4.2. Market Revenue and Forecast, by Application (2021-2033)
11.5.4.3. Market Revenue and Forecast, by Test Type (2021-2033)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Product (2021-2033)
11.5.5.2. Market Revenue and Forecast, by Application (2021-2033)
11.5.5.3. Market Revenue and Forecast, by Test Type (2021-2033)
Chapter 12. Company Profiles
12.1. Charles River Laboratories.
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. BSL Bioservice, Merck KGaA (MilliporeSigma).
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Samsung Biologics.
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Sartorius AG.
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Eurofins Scientific.
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. SGS Société Générale de Surveillance SA
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Thermo Fisher Scientific Inc..
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. BIOMÉRIEUX
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Lonza.
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others