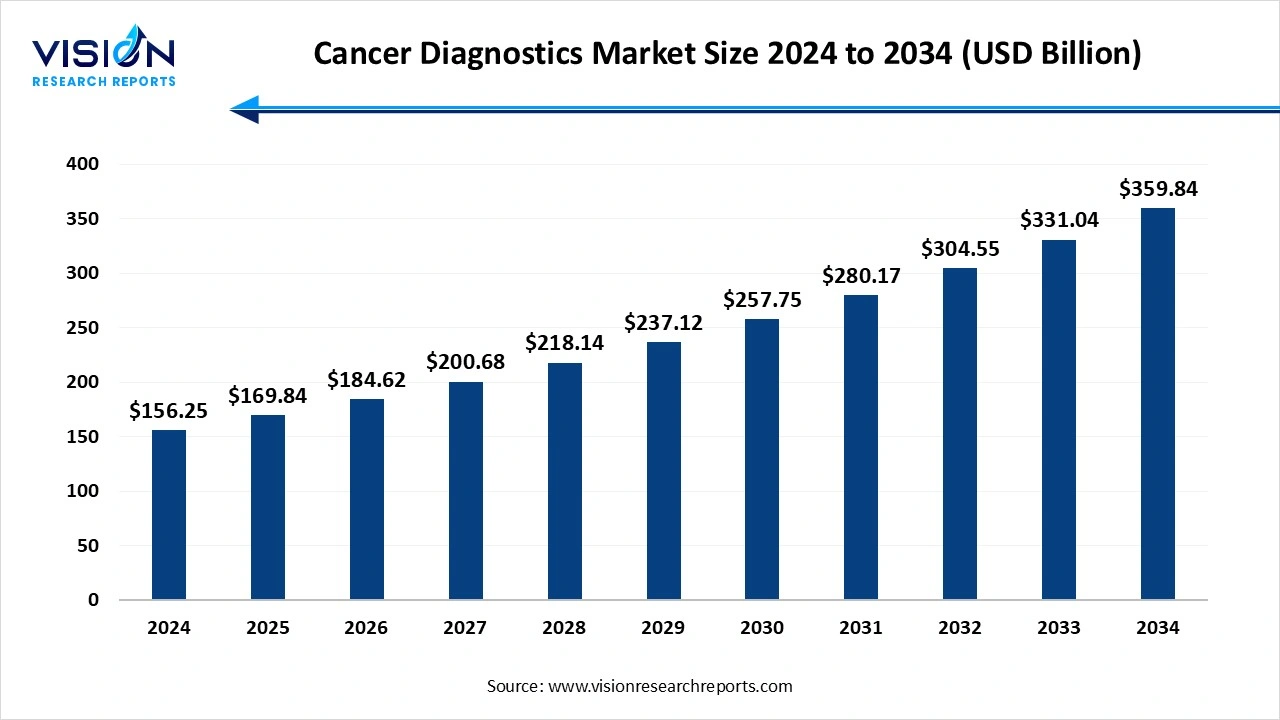
The global cancer diagnostics market size stood at USD 156.25 billion in 2024 and is estimated to reach USD 169.84 billion in 2025 It is projected to hit USD 359.84 billion by 2034, growing at a CAGR of 8.70% from 2025 to 2034.
The cancer diagnostics market is experiencing steady growth driven by rising cancer prevalence, advancements in diagnostic technologies, and increasing demand for early and accurate detection. Growing adoption of liquid biopsies, molecular diagnostics, AI-enabled imaging tools, and non-invasive screening methods is reshaping the landscape, enabling faster and more precise diagnosis. Government initiatives, improved healthcare infrastructure, and expanding clinical research activities are further accelerating market expansion. Additionally, the shift toward personalized medicine and biomarker-based testing continues to create new opportunities for innovative diagnostic solutions, positioning the market for strong growth over the coming years.
The cancer diagnostics market is propelled by several growth factors, including the escalating incidence of cancer worldwide, advancements in diagnostic technologies, and heightened awareness concerning early disease detection. Key players in the market are continually innovating and investing in research and development endeavors to introduce groundbreaking diagnostic solutions, thus enhancing precision and efficacy in cancer detection. Collaborations and partnerships between diagnostic companies and healthcare institutions are further augmenting market expansion and technological progression. Nonetheless, challenges such as the elevated costs associated with advanced diagnostic techniques, rigorous regulatory frameworks, and a shortage of skilled healthcare professionals remain significant hurdles to market growth.
| Report Coverage | Details |
| Market Size in 2024 | USD 156.25 Billion |
| Revenue Forecast by 2034 | USD 359.84 Billion |
| Growth rate from 2025 to 2034 | CAGR of 8.7% |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Regions | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Covered | Abbott, F. Hoffmann-La Roche Ltd., GE Healthcare, QIAGEN, BD, Koninklijke Philips N.V. (Philips), Siemens Healthcare GmbH, Hologic, Inc., Thermo Fisher Scientific, Inc., and Illumina, Inc. |
In 2024, North America emerged as the dominant force in the market, commanding a notable revenue share of 42%. The region's market dominance can be attributed to several factors, including the growing cancer burden in the United States and Canada. Additionally, there is a notable focus among medical device companies in the region on developing innovative diagnostic devices geared towards cancer detection, further propelling market growth. Product launches play a pivotal role in shaping market dynamics, exemplified by Nanostics Inc.'s announcement in July 2022 regarding the commencement of a clinical investigation for bladder cancer.
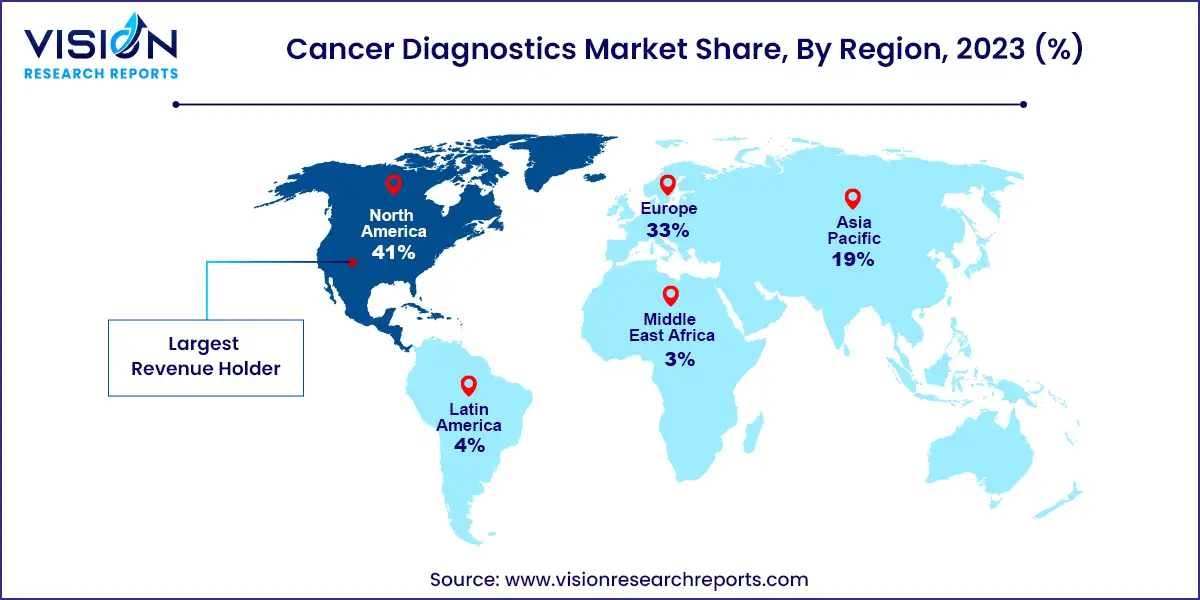 Conversely, the Asia Pacific market is poised to witness the fastest compound annual growth rate (CAGR) from 2025 to 2034, driven by various factors including increasing healthcare reforms. The region is experiencing a rise in cancer prevalence, particularly highlighted by a report published by PubMed Central in March 2022, which underscores the high incidence of cancer in China. With an estimated 4.8 million new cancer cases predicted in China by 2022, lung cancer emerges as the most prevalent type.
Conversely, the Asia Pacific market is poised to witness the fastest compound annual growth rate (CAGR) from 2025 to 2034, driven by various factors including increasing healthcare reforms. The region is experiencing a rise in cancer prevalence, particularly highlighted by a report published by PubMed Central in March 2022, which underscores the high incidence of cancer in China. With an estimated 4.8 million new cancer cases predicted in China by 2022, lung cancer emerges as the most prevalent type.
The consumables segment dominated the market with a share of 60% in 2024 and is poised to witness the most rapid growth throughout the forecast period. The development of advanced imaging diagnostic techniques and effective monoclonal antibody-based assays for detecting antigens and small chemicals produced by malignant cells holds significant promise for enhancing diagnostic capabilities. While monoclonal antibody (mAb) technology is still in its nascent stages, recent advancements in recombinant antigen synthesis and antibody creation techniques have substantially broadened its potential in diagnosis.
On the other hand, the instruments segment is expected to experience notable growth during the forecast period. Analytical instruments play a pivotal role in automating the diagnostic process and facilitating the convergence of samples and reagents. Kits and robots employed in pathology laboratories aid in the quantification and detection of infectious microorganisms, blood antigens, and viral load.
In 2024, the in vitro diagnostics (IVD) segment emerged as the dominant force, capturing a share of 53%, primarily fueled by the heightened adoption of IVD solutions amidst the COVID-19 pandemic. The surge in testing demand underscored the pivotal role of IVD in healthcare. The market is witnessing a shift towards automated IVD systems tailored for hospitals and laboratories, offering precise, efficient, and error-free diagnosis capabilities. Notably, in March 2019, BD obtained CE-IVD certification for its automated flow cytometry system, BD FACSDuet, empowering clinical laboratories to enhance efficacy and throughput compared to manual processes.
Meanwhile, laboratory diagnostic tests (LDTs) are poised to exhibit the highest compound annual growth rate (CAGR) from 2025 to 2034. These tests serve as critical tools for swiftly integrating new information and guiding personalized therapy decisions. By enabling treatments to pivot from a generalized approach to a more tailored one based on individual genetic profiles and disease characteristics, LDTs are revolutionizing patient care. Notably, LDTs are introduced to the market without independent regulatory assessment or FDA approval, as they are generated and utilized within the same facility.
In 2024, the breast cancer segment emerged as the leader in revenue, capturing a substantial share of 15%. This dominance is poised to continue, driven by increased research and development efforts aimed at advancing screening tools for breast cancer detection. Notably, a groundbreaking research study published in Scientific Reports in September 2019 introduced a novel screening test. This test combines the expression of MMP-1 (matrix metalloproteinase-1) and miR-21 in urinary exosomes, enabling the detection of 95% of breast cancers without metastasis development.
The lung cancer segment is expected to experience the fastest growth within the cancer diagnostics market. Diagnosis of lung cancer often relies on advanced imaging tools, including computed tomography (CT), positron emission tomography (PET), and magnetic resonance imaging (MRI). Given the complexity of lung cancer characterized by multiple causes, diverse symptoms, and various tumor types accurate diagnosis is essential for effective treatment and a favorable prognosis. Medical centers with extensive experience in detecting and managing lung cancer are better positioned to identify the underlying causes of symptoms and provide precise, targeted care.
In 2024, the laboratories segment emerged as the dominant force in the market, commanding a significant share of 54%. This segment is poised for substantial growth over the forecast period, primarily driven by the increasing demand for testing services and the availability of resources to conduct these tests. Diagnostic laboratories are experiencing heightened reliance from hospitals for testing and evaluation purposes, thereby propelling accelerated growth. Several factors contribute to the segment's expansion, including heightened awareness about personalized medicine, advancements in technology, and a growing demand for cost-effective services. Additionally, government initiatives aimed at providing facilities such as compensation for diagnostic tests are further fueling growth. Regulatory authorities are also taking proactive steps to enhance clinical laboratory diagnostic services and streamline the diagnostic process.
Conversely, the others segment, encompassing home-based tests, is projected to exhibit the fastest compound annual growth rate (CAGR) of 7.18% from 2024 to 2033. This surge is attributed to the increased adoption of home-based and technologically advanced treatments, particularly amid the COVID-19 pandemic. Acknowledging the need for new models in the cancer community and among payers to mitigate unscheduled hospitalizations and emergency department visits, acute home-based treatment presents a promising alternative to traditional care settings. Key market players are focusing on developing home-based tests to enhance accessibility and acceptability, further driving demand within the segment.
The Panel-based tests and BRCA1/2 genetic testing serve distinct yet complementary roles in assessing the inherited risk of breast and ovarian cancers. Panel-based tests offer broader coverage, detecting genetic mutations associated with a range of hereditary cancer syndromes beyond BRCA1/2, such as pheochromocytoma-paraganglioma and colon cancer. This expanded clinical value and yield make panel-based testing particularly valuable for individuals with diverse cancer risks. Moreover, patients with genetic abnormalities linked to hereditary cancer syndromes often exhibit an earlier onset of certain cancers, necessitating early detection measures. Consequently, the demand for panel tests is driven by the imperative to provide timely detection and intervention.
On the other hand, biopsy procedures are projected to witness the fastest compound annual growth rate (CAGR) from 2024 to 2033, owing to their numerous benefits, including early cancer detection and characterization. Biopsies not only facilitate the identification of cancer but also enable assessment of its aggressiveness and propensity for metastasis. This information is crucial in devising tailored treatment strategies based on the cancer's stage and behavior. Notably, product launches play a pivotal role in driving market growth.
By Product
By Type
By Application
By End-use
By Test Type
By Region
Life Science Market
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Life Science Market
5.1. COVID-19 Landscape: Life Science Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market TrTest Types and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and TrTest Types
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. VTest Typeor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Cancer Diagnostics Market, By Product
8.1. Cancer Diagnostics Market, by Product
8.1.1. Instruments
8.1.1.1. Market Revenue and Forecast
8.1.2. Consumables
8.1.2.1. Market Revenue and Forecast
8.1.3. Services
8.1.3.1. Market Revenue and Forecast
Chapter 9. Global Cancer Diagnostics Market, By Type
9.1. Cancer Diagnostics Market, by Type
9.1.1. IVD
9.1.1.1. Market Revenue and Forecast
9.1.2. LDT
9.1.2.1. Market Revenue and Forecast
9.1.3. Imaging
9.1.3.1. Market Revenue and Forecast
Chapter 10. Global Cancer Diagnostics Market, By Application
10.1. Cancer Diagnostics Market, by Application
10.1.1. Breast Cancer
10.1.1.1. Market Revenue and Forecast
10.1.2. Colorectal Cancer
10.1.2.1. Market Revenue and Forecast
10.1.3. Cervical Cancer
10.1.3.1. Market Revenue and Forecast
10.1.4. Lung Cancer
10.1.4.1. Market Revenue and Forecast
10.1.5. Prostate Cancer
10.1.5.1. Market Revenue and Forecast
10.1.6. Skin Cancer
10.1.6.1. Market Revenue and Forecast
10.1.7. Blood Cancer
10.1.7.1. Market Revenue and Forecast
10.1.8. Kidney Cancer
10.1.8.1. Market Revenue and Forecast
10.1.9. Liver Cancer
10.1.9.1. Market Revenue and Forecast
10.1.10. Pancreatic Cancer
10.1.10.1. Market Revenue and Forecast
10.1.11. Ovarian Cancer
10.1.11.1. Market Revenue and Forecast
Chapter 11. Global Cancer Diagnostics Market, By End-use
11.1. Cancer Diagnostics Market, by End-use
11.1.1. Hospitals
11.1.1.1. Market Revenue and Forecast
11.1.2. Laboratories
11.1.2.1. Market Revenue and Forecast
11.1.3. Others
11.1.3.1. Market Revenue and Forecast
Chapter 12. Global Life Science Market, By Test Type
12.1. Life Science Market, by Test Type
12.1.1. Biopsy
12.1.1.1. Market Revenue and Forecast
12.1.2. Others
12.1.2.1. Market Revenue and Forecast
Chapter 13. Global Life Science Market, Regional Estimates and TrTest Type Forecast
13.1. North America
13.1.1. Market Revenue and Forecast, by Product Type
13.1.2. Market Revenue and Forecast, by Technology
13.1.3. Market Revenue and Forecast, by March Mode
13.1.4. Market Revenue and Forecast, by April Size
13.1.5. Market Revenue and Forecast, by Test Type Use
13.1.6. U.S.
13.1.6.1. Market Revenue and Forecast, by Product Type
13.1.6.2. Market Revenue and Forecast, by Technology
13.1.6.3. Market Revenue and Forecast, by March Mode
13.1.6.4. Market Revenue and Forecast, by April Size
13.1.7. Market Revenue and Forecast, by Test Type Use
13.1.8. Rest of North America
13.1.8.1. Market Revenue and Forecast, by Product Type
13.1.8.2. Market Revenue and Forecast, by Technology
13.1.8.3. Market Revenue and Forecast, by March
13.1.8.4. Market Revenue and Forecast, by April
13.1.8.5. Market Revenue and Forecast, by Test Type
13.2. Europe
13.2.1. Market Revenue and Forecast, by Product Type
13.2.2. Market Revenue and Forecast, by Technology
13.2.3. Market Revenue and Forecast, by March
13.2.4. Market Revenue and Forecast, by April
13.2.5. Market Revenue and Forecast, by Test Type
13.2.6. UK
13.2.6.1. Market Revenue and Forecast, by Product Type
13.2.6.2. Market Revenue and Forecast, by Technology
13.2.6.3. Market Revenue and Forecast, by March
13.2.7. Market Revenue and Forecast, by April
13.2.8. Market Revenue and Forecast, by Test Type
13.2.9. Germany
13.2.9.1. Market Revenue and Forecast, by Product Type
13.2.9.2. Market Revenue and Forecast, by Technology
13.2.9.3. Market Revenue and Forecast, by March
13.2.10. Market Revenue and Forecast, by April
13.2.11. Market Revenue and Forecast, by Test Type
13.2.12. France
13.2.12.1. Market Revenue and Forecast, by Product Type
13.2.12.2. Market Revenue and Forecast, by Technology
13.2.12.3. Market Revenue and Forecast, by March
13.2.12.4. Market Revenue and Forecast, by April
13.2.13. Market Revenue and Forecast, by Test Type
13.2.14. Rest of Europe
13.2.14.1. Market Revenue and Forecast, by Product Type
13.2.14.2. Market Revenue and Forecast, by Technology
13.2.14.3. Market Revenue and Forecast, by March
13.2.14.4. Market Revenue and Forecast, by April
13.2.15. Market Revenue and Forecast, by Test Type
13.3. APAC
13.3.1. Market Revenue and Forecast, by Product Type
13.3.2. Market Revenue and Forecast, by Technology
13.3.3. Market Revenue and Forecast, by March
13.3.4. Market Revenue and Forecast, by April
13.3.5. Market Revenue and Forecast, by Test Type
13.3.6. India
13.3.6.1. Market Revenue and Forecast, by Product Type
13.3.6.2. Market Revenue and Forecast, by Technology
13.3.6.3. Market Revenue and Forecast, by March
13.3.6.4. Market Revenue and Forecast, by April
13.3.7. Market Revenue and Forecast, by Test Type
13.3.8. China
13.3.8.1. Market Revenue and Forecast, by Product Type
13.3.8.2. Market Revenue and Forecast, by Technology
13.3.8.3. Market Revenue and Forecast, by March
13.3.8.4. Market Revenue and Forecast, by April
13.3.9. Market Revenue and Forecast, by Test Type
13.3.10. Japan
13.3.10.1. Market Revenue and Forecast, by Product Type
13.3.10.2. Market Revenue and Forecast, by Technology
13.3.10.3. Market Revenue and Forecast, by March
13.3.10.4. Market Revenue and Forecast, by April
13.3.10.5. Market Revenue and Forecast, by Test Type
13.3.11. Rest of APAC
13.3.11.1. Market Revenue and Forecast, by Product Type
13.3.11.2. Market Revenue and Forecast, by Technology
13.3.11.3. Market Revenue and Forecast, by March
13.3.11.4. Market Revenue and Forecast, by April
13.3.11.5. Market Revenue and Forecast, by Test Type
13.4. MEA
13.4.1. Market Revenue and Forecast, by Product Type
13.4.2. Market Revenue and Forecast, by Technology
13.4.3. Market Revenue and Forecast, by March
13.4.4. Market Revenue and Forecast, by April
13.4.5. Market Revenue and Forecast, by Test Type
13.4.6. GCC
13.4.6.1. Market Revenue and Forecast, by Product Type
13.4.6.2. Market Revenue and Forecast, by Technology
13.4.6.3. Market Revenue and Forecast, by March
13.4.6.4. Market Revenue and Forecast, by April
13.4.7. Market Revenue and Forecast, by Test Type
13.4.8. North Africa
13.4.8.1. Market Revenue and Forecast, by Product Type
13.4.8.2. Market Revenue and Forecast, by Technology
13.4.8.3. Market Revenue and Forecast, by March
13.4.8.4. Market Revenue and Forecast, by April
13.4.9. Market Revenue and Forecast, by Test Type
13.4.10. South Africa
13.4.10.1. Market Revenue and Forecast, by Product Type
13.4.10.2. Market Revenue and Forecast, by Technology
13.4.10.3. Market Revenue and Forecast, by March
13.4.10.4. Market Revenue and Forecast, by April
13.4.10.5. Market Revenue and Forecast, by Test Type
13.4.11. Rest of MEA
13.4.11.1. Market Revenue and Forecast, by Product Type
13.4.11.2. Market Revenue and Forecast, by Technology
13.4.11.3. Market Revenue and Forecast, by March
13.4.11.4. Market Revenue and Forecast, by April
13.4.11.5. Market Revenue and Forecast, by Test Type
13.5. Latin America
13.5.1. Market Revenue and Forecast, by Product Type
13.5.2. Market Revenue and Forecast, by Technology
13.5.3. Market Revenue and Forecast, by March
13.5.4. Market Revenue and Forecast, by April
13.5.5. Market Revenue and Forecast, by Test Type
13.5.6. Brazil
13.5.6.1. Market Revenue and Forecast, by Product Type
13.5.6.2. Market Revenue and Forecast, by Technology
13.5.6.3. Market Revenue and Forecast, by March
13.5.6.4. Market Revenue and Forecast, by April
13.5.7. Market Revenue and Forecast, by Test Type
13.5.8. Rest of LATAM
13.5.8.1. Market Revenue and Forecast, by Product Type
13.5.8.2. Market Revenue and Forecast, by Technology
13.5.8.3. Market Revenue and Forecast, by March
13.5.8.4. Market Revenue and Forecast, by April
13.5.8.5. Market Revenue and Forecast, by Test Type
Chapter 14. Company Profiles
14.1. Abbott
14.1.1. Company Overview
14.1.2. Product Offerings
14.1.3. Financial Performance
14.1.4. Recent Initiatives
14.2. F. Hoffmann-La Roche Ltd.
14.2.1. Company Overview
14.2.2. Product Offerings
14.2.3. Financial Performance
14.2.4. Recent Initiatives
14.3. GE Healthcare
14.3.1. Company Overview
14.3.2. Product Offerings
14.3.3. Financial Performance
14.3.4. Recent Initiatives
14.4. QIAGEN
14.4.1. Company Overview
14.4.2. Product Offerings
14.4.3. Financial Performance
14.4.4. Recent Initiatives
14.5. BD
14.5.1. Company Overview
14.5.2. Product Offerings
14.5.3. Financial Performance
14.5.4. Recent Initiatives
14.6. Koninklijke Philips N.V. (Philips)
14.6.1. Company Overview
14.6.2. Product Offerings
14.6.3. Financial Performance
14.6.4. Recent Initiatives
14.7. Siemens Healthcare GmbH
14.7.1. Company Overview
14.7.2. Product Offerings
14.7.3. Financial Performance
14.7.4. Recent Initiatives
14.8. Hologic, Inc
14.8.1. Company Overview
14.8.2. Product Offerings
14.8.3. Financial Performance
14.8.4. Recent Initiatives
14.9. Thermo Fisher Scientific, Inc.
14.9.1. Company Overview
14.9.2. Product Offerings
14.9.3. Financial Performance
14.9.4. Recent Initiatives
14.10. Illumina, Inc.
14.10.1. Company Overview
14.10.2. Product Offerings
14.10.3. Financial Performance
14.10.4. Recent Initiatives
Chapter 15. Research Methodology
15.1. Primary Research
15.2. Secondary Research
15.3. Assumptions
Chapter 16. AppTest Typeix
16.1. About Us
16.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others