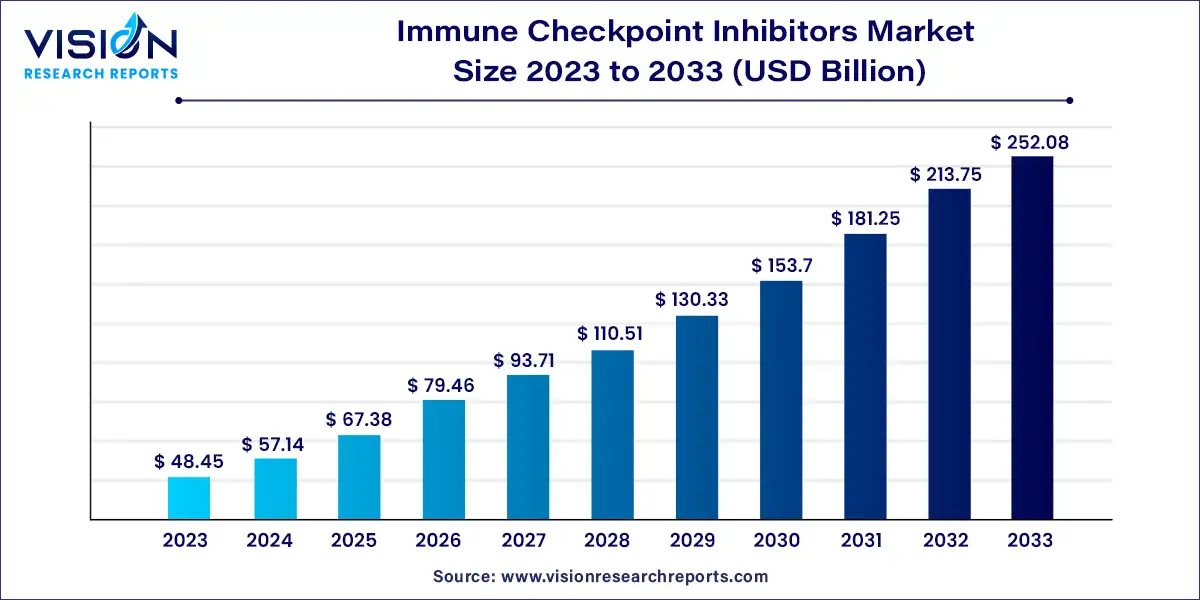
The global immune checkpoint inhibitors market size was estimated at around USD 48.45 billion in 2023 and it is projected to hit around USD 252.08 billion by 2033, growing at a CAGR of 17.93% from 2024 to 2033. Immune checkpoint inhibitors have revolutionized cancer treatment by harnessing the body’s immune system to target and destroy cancer cells. These therapies have gained prominence due to their ability to provide durable responses and improve survival rates in various cancers. The immune checkpoint inhibitors market has experienced significant growth, driven by ongoing research, clinical trials, and an increasing number of approved therapies.
The growth of the immune checkpoint inhibitors market is largely driven by an escalating global incidence of cancer is a primary catalyst, as these therapies offer significant promise for treating a range of malignancies. Additionally, rapid advancements in research and development contribute to the market's expansion by enabling the creation of novel and more effective immune checkpoint inhibitors. The continuous approval of new drugs for diverse cancer types further propels market growth, as it opens new treatment avenues and increases the accessibility of these therapies. Moreover, heightened awareness among both healthcare professionals and patients about the efficacy of immune checkpoint inhibitors enhances their adoption, fostering market expansion. Together, these factors create a dynamic environment conducive to the sustained growth of the immune checkpoint inhibitors market.
North America dominated the immune checkpoint inhibitors market with a 63% revenue share in 2023, driven by the high prevalence of cancers in the region. For instance, the Leukemia & Lymphoma Society reports that a person in the U.S. is diagnosed with lymphoma, myeloma, or leukemia every three minutes. Additionally, the CDC reported approximately 1,603,844 new cancer cases in the U.S. in 2020. The expansion of immunotherapy centers, such as the James P. Allison Institute launched by The University of Texas MD Anderson Cancer Center in March 2022, and increased FDA approvals for novel therapies, such as Regeneron Pharmaceuticals Inc.'s Libtayo (cemiplimab-rwlc) for non-small cell lung cancer, are driving market growth.
| Attribute | North America |
| Market Value | USD 30.52 Billion |
| Growth Rate | 17.94% CAGR |
| Projected Value | USD 158.81 Billion |
The immune checkpoint inhibitors market in Europe is expected to grow at a significant rate during the forecast period. This growth is driven by increasing cancer prevalence, demand for efficient treatments, a well-developed healthcare infrastructure, and a focus on early disease detection.

The Asia Pacific market is anticipated to witness significant growth due to factors such as healthcare reforms, improving healthcare infrastructure, a growing population, and the entry of local companies into the market. The region has a large population and high cancer prevalence, with an estimated 10.5 million new cancer cases in 2022 according to Global Cancer Statistics. The focus on developing and approving new immune checkpoint inhibitors is expected to drive market growth in Asia Pacific.
In 2023, the PD-1 segment dominated the market, accounting for a substantial 74% of the revenue share. PD-1 inhibitors are effective across a range of cancers, including melanoma, lung cancer, and bladder cancer, owing to their ability to produce durable clinical responses. Their broad applicability and effectiveness drive widespread adoption, further supported by their use in combination with other therapies such as chemotherapy and CTLA-4 inhibitors. The market is poised for growth due to ongoing product innovations and approvals. For instance, in February 2023, BeiGene LTD. received approval from the China National Medical Products Administration (NMPA) for Tislelizumab in combination with platinum-based chemotherapy and fluoropyrimidine. This combination is particularly effective for patients with advanced or metastatic gastric cancer.
The PD-L1 segment is projected to grow at the fastest rate during the forecast period. PD-L1 inhibitors like Atezolizumab (Tecentriq), Avelumab (Bavencio), and Durvalumab (Imfinzi) are becoming increasingly popular due to their high efficacy. These inhibitors are versatile, used either alone or in combination for treating various cancers, including non-small cell lung cancer and metastatic Merkel cell carcinoma.
Lung cancer led the market with a revenue share of 26% in 2023. It remains the leading cause of cancer-related deaths globally, with cases expected to rise from 2.48 million in 2022 to 3.05 million by 2030, according to GLOBOCAN. The high prevalence and mortality rates of lung cancer drive the demand for immune checkpoint inhibitors. Recent approvals, such as Merck & Co., Inc.'s KEYTRUDA (pembrolizumab) for patients with stage IB, II, and IIIA non-small cell lung cancer, underscore the significant role of immune checkpoint inhibitors in this segment.
The colorectal cancer segment is anticipated to experience significant growth. CRC is the third-most common cancer globally, with about 1 in 10 cancer patients affected. It is also the second-leading cause of cancer-related deaths worldwide. The rising incidence of CRC drives demand for effective treatments. Merck & Co., Inc.'s KEYTRUDA (pembrolizumab) received FDA approval in June 2020 for treating metastatic colorectal cancer, reflecting ongoing efforts to address this critical need.
In 2023, hospital pharmacies held the largest revenue share of 57%. Immune checkpoint inhibitors are predominantly administered in hospital settings due to their complex nature and the availability of comprehensive care. The collaboration between pharmaceutical companies, academic institutions, and hospitals facilitates access to clinical trials and early adoption of new therapies. For example, a 2023 study supported by AstraZeneca and the National Institute of Cancer highlighted the potential of durvalumab in treating non-small cell lung cancer, showcasing the importance of hospital pharmacies in cancer care.
The online pharmacies segment is expected to grow at the fastest rate. Online platforms provide a convenient and accessible option for patients to obtain immune checkpoint inhibitors. The rise in digital health solutions and telemedicine has fueled this growth, allowing patients to manage prescriptions and access medications from home. The increasing global internet accessibility and patient preference for virtual healthcare are likely to drive the expansion of online pharmacies.
By Type
By Application
By Distribution Channel
By Region
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Immune Checkpoint Inhibitors Market
5.1. COVID-19 Landscape: Immune Checkpoint Inhibitors Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Immune Checkpoint Inhibitors Market, By Type
8.1. Immune Checkpoint Inhibitors Market, by Type, 2024-2033
8.1.1. CTLA-4 Inhibitor
8.1.1.1. Market Revenue and Forecast (2021-2033)
8.1.2. PD-1 Inhibitor
8.1.2.1. Market Revenue and Forecast (2021-2033)
8.1.3. PD-L1 Inhibitor
8.1.3.1. Market Revenue and Forecast (2021-2033)
Chapter 9. Global Immune Checkpoint Inhibitors Market, By Application
9.1. Immune Checkpoint Inhibitors Market, by Application, 2024-2033
9.1.1. Lung Cancer
9.1.1.1. Market Revenue and Forecast (2021-2033)
9.1.2. Bladder Cancer
9.1.2.1. Market Revenue and Forecast (2021-2033)
9.1.3. Melanoma
9.1.3.1. Market Revenue and Forecast (2021-2033)
9.1.4. Hodgkin lymphoma
9.1.4.1. Market Revenue and Forecast (2021-2033)
9.1.5. Others
9.1.5.1. Market Revenue and Forecast (2021-2033)
Chapter 10. Global Immune Checkpoint Inhibitors Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Type (2021-2033)
10.1.2. Market Revenue and Forecast, by Application (2021-2033)
10.1.3. U.S.
10.1.3.1. Market Revenue and Forecast, by Type (2021-2033)
10.1.3.2. Market Revenue and Forecast, by Application (2021-2033)
10.1.4. Rest of North America
10.1.4.1. Market Revenue and Forecast, by Type (2021-2033)
10.1.4.2. Market Revenue and Forecast, by Application (2021-2033)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Type (2021-2033)
10.2.2. Market Revenue and Forecast, by Application (2021-2033)
10.2.3. UK
10.2.3.1. Market Revenue and Forecast, by Type (2021-2033)
10.2.3.2. Market Revenue and Forecast, by Application (2021-2033)
10.2.4. Germany
10.2.4.1. Market Revenue and Forecast, by Type (2021-2033)
10.2.4.2. Market Revenue and Forecast, by Application (2021-2033)
10.2.5. France
10.2.5.1. Market Revenue and Forecast, by Type (2021-2033)
10.2.5.2. Market Revenue and Forecast, by Application (2021-2033)
10.2.6. Rest of Europe
10.2.6.1. Market Revenue and Forecast, by Type (2021-2033)
10.2.6.2. Market Revenue and Forecast, by Application (2021-2033)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Type (2021-2033)
10.3.2. Market Revenue and Forecast, by Application (2021-2033)
10.3.3. India
10.3.3.1. Market Revenue and Forecast, by Type (2021-2033)
10.3.3.2. Market Revenue and Forecast, by Application (2021-2033)
10.3.4. China
10.3.4.1. Market Revenue and Forecast, by Type (2021-2033)
10.3.4.2. Market Revenue and Forecast, by Application (2021-2033)
10.3.5. Japan
10.3.5.1. Market Revenue and Forecast, by Type (2021-2033)
10.3.5.2. Market Revenue and Forecast, by Application (2021-2033)
10.3.6. Rest of APAC
10.3.6.1. Market Revenue and Forecast, by Type (2021-2033)
10.3.6.2. Market Revenue and Forecast, by Application (2021-2033)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Type (2021-2033)
10.4.2. Market Revenue and Forecast, by Application (2021-2033)
10.4.3. GCC
10.4.3.1. Market Revenue and Forecast, by Type (2021-2033)
10.4.3.2. Market Revenue and Forecast, by Application (2021-2033)
10.4.4. North Africa
10.4.4.1. Market Revenue and Forecast, by Type (2021-2033)
10.4.4.2. Market Revenue and Forecast, by Application (2021-2033)
10.4.5. South Africa
10.4.5.1. Market Revenue and Forecast, by Type (2021-2033)
10.4.5.2. Market Revenue and Forecast, by Application (2021-2033)
10.4.6. Rest of MEA
10.4.6.1. Market Revenue and Forecast, by Type (2021-2033)
10.4.6.2. Market Revenue and Forecast, by Application (2021-2033)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Type (2021-2033)
10.5.2. Market Revenue and Forecast, by Application (2021-2033)
10.5.3. Brazil
10.5.3.1. Market Revenue and Forecast, by Type (2021-2033)
10.5.3.2. Market Revenue and Forecast, by Application (2021-2033)
10.5.4. Rest of LATAM
10.5.4.1. Market Revenue and Forecast, by Type (2021-2033)
10.5.4.2. Market Revenue and Forecast, by Application (2021-2033)
Chapter 11. Company Profiles
11.1. AstraZeneca PLC
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Bristol-Myers Squibb Company
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Eli Lilly and Company (ARMO Biosciences.)
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. GlaxoSmithKline PLC
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. Recent Initiatives
11.5. F. Hoffmann-La Roche Ltd. (Genentech Inc.,)
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Sanofi
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Merck & Co., Inc.
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Merck KGaA (EMD Serono Inc.)
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. BeiGene Ltd
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. Shanghai Jhunsi Biosciences Ltd.
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others