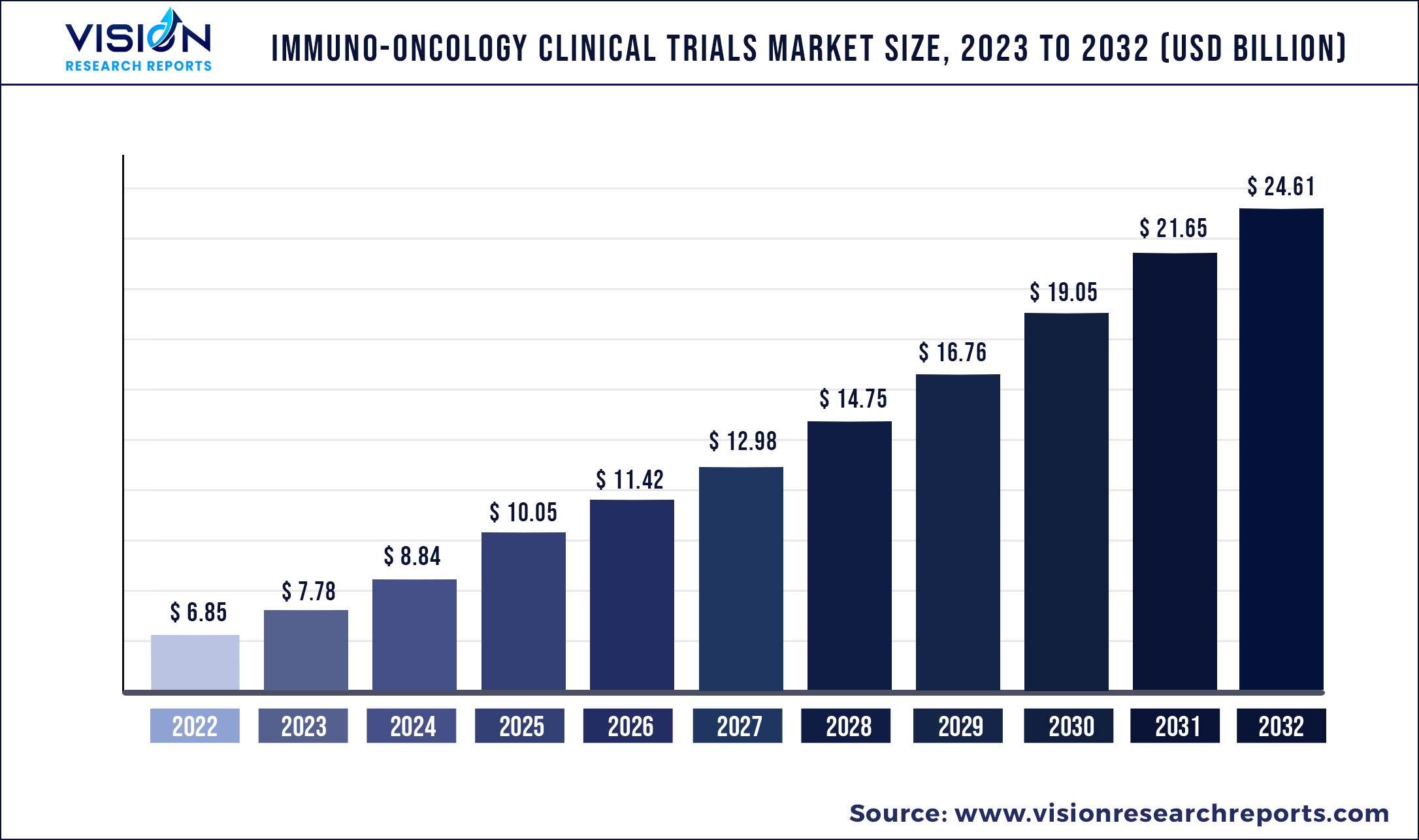
The global immuno-oncology clinical trials market was valued at USD 6.85 billion in 2022 and it is predicted to surpass around USD 24.61 billion by 2032 with a CAGR of 13.65% from 2023 to 2032.
Key Pointers
Report Scope of the Immuno-oncology Clinical Trials Market
| Report Coverage | Details |
| Market Size in 2022 | USD 6.85 billion |
| Revenue Forecast by 2032 | USD 24.61 billion |
| Growth rate from 2023 to 2032 | CAGR of 13.65% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Market Analysis (Terms Used) | Value (US$ Million/Billion) or (Volume/Units) |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
The growth can be attributed to recent developments in immuno-oncology, changes in lifestyle brought on by urbanization predisposing populations to the rise of cancer, and the growing acceptability of targeted therapy.
COVID-19 has had a significant impact on cancer and clinical trials, altering therapy and oncology patients in a number of ways. According to the research by Evaluate Vantage, over 170 studies being discontinued as a result of the virus. The pandemic disrupted 920 interventional oncology trials between February and May 2020.
Besides, clinical trial participation has also become challenging due to difficulties such as transportation issues to trial sites or testing locations. IQVIA and the Cancer Research Institute collaborated to conduct a survey among oncology investigators to assess the impact of COVID-19 on ongoing and future clinical studies. Remote tools were highly ranked for the assessments. Also, during the COVID-19 pandemic, several regional authorities provided prompt and detailed support for virtual tools and models, indicating that regulators regard patient-centric technology and approaches as feasible answers to this global issue.
Immuno-oncology trials account for more than a third of all clinical trials in oncology. Small biotech and specialty pharma are poised to play a critical role in the growth. Immunotherapy advances differ from cytotoxic chemotherapy in the way they work, and these distinctions may have an influence on a dose, response evaluation, biomarker validation, combination therapy selection, and adverse event detection. Understanding and overcoming these barriers will be crucial to the success of immuno-oncology clinical trials and, eventually, market approval.
Treatments focusing on two targets - PD-1/PD-L1 and CTLA-4 - which both negatively control T-cell immune function to promote activation of the body's own immune system - have generated the most significant advances in immuno-oncology. Other than PD-1/PD-L1 and CTLA-4 inhibitors, CAR-T (chimeric antigen receptor T-cell) treatment is another popular immuno-oncology treatment option. CAR-T therapies use a patient's own T-cells, which are extracted, changed to attack cancer, and then put back into the patient's body.
Immuno-oncology (IO) continues to play a significant role in oncology deal-making, accounting for 49% of drug licensing deals and 66% of the total disclosed deal value. 15 of the 21 oncology medication licensing deals worth more than $1 billion were for IO assets, with 9 of those for multi-targeted methods such bispecific antibodies and antibody-drug conjugates. In 2020, the AstraZeneca/Daiichi Sankyo pact was the largest drug licensing deal.
Key Companies & Market Share Insights
Key players in the market are undertaking various strategic initiatives such as the signing of the new partnership agreement, collaborations, mergers and acquisitions, geographic expansion, aiming to strengthen their product portfolio, manufacturing capacities, thus providing a competitive advantage.
For example, in May 2021, BioNTech announced its plans to open its first mRNA manufacturing facility in Singapore which will expand its geographical footprint in Southeast Asia. The new mRNA production plant would take advantage of cutting-edge technology and digital infrastructure. Some of the prominent players in the immuno-oncology clinical trials market include:
Immuno-oncology Clinical Trials Market Segmentations:
| By Phase | By Design | By Indication |
|
Phase I Phase II Phase III Phase IV |
Interventional trials Observational trials Expanded access trials |
Solid tumors Hematological cancer |
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Phase Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Immuno-oncology Clinical Trials Market
5.1. COVID-19 Landscape: Immuno-oncology Clinical Trials Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Immuno-oncology Clinical Trials Market, By Phase
8.1. Immuno-oncology Clinical Trials Market, by Phase, 2023-2032
8.1.1 Phase I
8.1.1.1. Market Revenue and Forecast (2020-2032)
8.1.2. Phase II
8.1.2.1. Market Revenue and Forecast (2020-2032)
8.1.3. Phase III
8.1.3.1. Market Revenue and Forecast (2020-2032)
8.1.4. Phase IV
8.1.4.1. Market Revenue and Forecast (2020-2032)
Chapter 9. Global Immuno-oncology Clinical Trials Market, By Design
9.1. Immuno-oncology Clinical Trials Market, by Design, 2023-2032
9.1.1. Interventional trials
9.1.1.1. Market Revenue and Forecast (2020-2032)
9.1.2. Observational trials
9.1.2.1. Market Revenue and Forecast (2020-2032)
9.1.3. Expanded access trials
9.1.3.1. Market Revenue and Forecast (2020-2032)
Chapter 10. Global Immuno-oncology Clinical Trials Market, By Indication
10.1. Immuno-oncology Clinical Trials Market, by Indication, 2023-2032
10.1.1. Solid tumors
10.1.1.1. Market Revenue and Forecast (2020-2032)
10.1.2. Hematological cancer
10.1.2.1. Market Revenue and Forecast (2020-2032)
Chapter 11. Global Immuno-oncology Clinical Trials Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Phase (2020-2032)
11.1.2. Market Revenue and Forecast, by Design (2020-2032)
11.1.3. Market Revenue and Forecast, by Indication (2020-2032)
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Phase (2020-2032)
11.1.4.2. Market Revenue and Forecast, by Design (2020-2032)
11.1.4.3. Market Revenue and Forecast, by Indication (2020-2032)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Phase (2020-2032)
11.1.5.2. Market Revenue and Forecast, by Design (2020-2032)
11.1.5.3. Market Revenue and Forecast, by Indication (2020-2032)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Phase (2020-2032)
11.2.2. Market Revenue and Forecast, by Design (2020-2032)
11.2.3. Market Revenue and Forecast, by Indication (2020-2032)
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Phase (2020-2032)
11.2.4.2. Market Revenue and Forecast, by Design (2020-2032)
11.2.4.3. Market Revenue and Forecast, by Indication (2020-2032)
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Phase (2020-2032)
11.2.5.2. Market Revenue and Forecast, by Design (2020-2032)
11.2.5.3. Market Revenue and Forecast, by Indication (2020-2032)
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Phase (2020-2032)
11.2.6.2. Market Revenue and Forecast, by Design (2020-2032)
11.2.6.3. Market Revenue and Forecast, by Indication (2020-2032)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Phase (2020-2032)
11.2.7.2. Market Revenue and Forecast, by Design (2020-2032)
11.2.7.3. Market Revenue and Forecast, by Indication (2020-2032)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Phase (2020-2032)
11.3.2. Market Revenue and Forecast, by Design (2020-2032)
11.3.3. Market Revenue and Forecast, by Indication (2020-2032)
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Phase (2020-2032)
11.3.4.2. Market Revenue and Forecast, by Design (2020-2032)
11.3.4.3. Market Revenue and Forecast, by Indication (2020-2032)
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Phase (2020-2032)
11.3.5.2. Market Revenue and Forecast, by Design (2020-2032)
11.3.5.3. Market Revenue and Forecast, by Indication (2020-2032)
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Phase (2020-2032)
11.3.6.2. Market Revenue and Forecast, by Design (2020-2032)
11.3.6.3. Market Revenue and Forecast, by Indication (2020-2032)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Phase (2020-2032)
11.3.7.2. Market Revenue and Forecast, by Design (2020-2032)
11.3.7.3. Market Revenue and Forecast, by Indication (2020-2032)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Phase (2020-2032)
11.4.2. Market Revenue and Forecast, by Design (2020-2032)
11.4.3. Market Revenue and Forecast, by Indication (2020-2032)
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Phase (2020-2032)
11.4.4.2. Market Revenue and Forecast, by Design (2020-2032)
11.4.4.3. Market Revenue and Forecast, by Indication (2020-2032)
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Phase (2020-2032)
11.4.5.2. Market Revenue and Forecast, by Design (2020-2032)
11.4.5.3. Market Revenue and Forecast, by Indication (2020-2032)
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Phase (2020-2032)
11.4.6.2. Market Revenue and Forecast, by Design (2020-2032)
11.4.6.3. Market Revenue and Forecast, by Indication (2020-2032)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Phase (2020-2032)
11.4.7.2. Market Revenue and Forecast, by Design (2020-2032)
11.4.7.3. Market Revenue and Forecast, by Indication (2020-2032)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Phase (2020-2032)
11.5.2. Market Revenue and Forecast, by Design (2020-2032)
11.5.3. Market Revenue and Forecast, by Indication (2020-2032)
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Phase (2020-2032)
11.5.4.2. Market Revenue and Forecast, by Design (2020-2032)
11.5.4.3. Market Revenue and Forecast, by Indication (2020-2032)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Phase (2020-2032)
11.5.5.2. Market Revenue and Forecast, by Design (2020-2032)
11.5.5.3. Market Revenue and Forecast, by Indication (2020-2032)
Chapter 12. Company Profiles
12.1. Medpace
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Novartis
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Exscientia
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Syneous Health
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. AstraZeneca
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others