The global liver cancer drug market size was valued at USD 4.42 billion in 2025 and is expected to grow from USD 5.22 billion in 2026 to reach USD 23.53 billion by 2034, expanding at a CAGR of 18.2% during the forecast period.
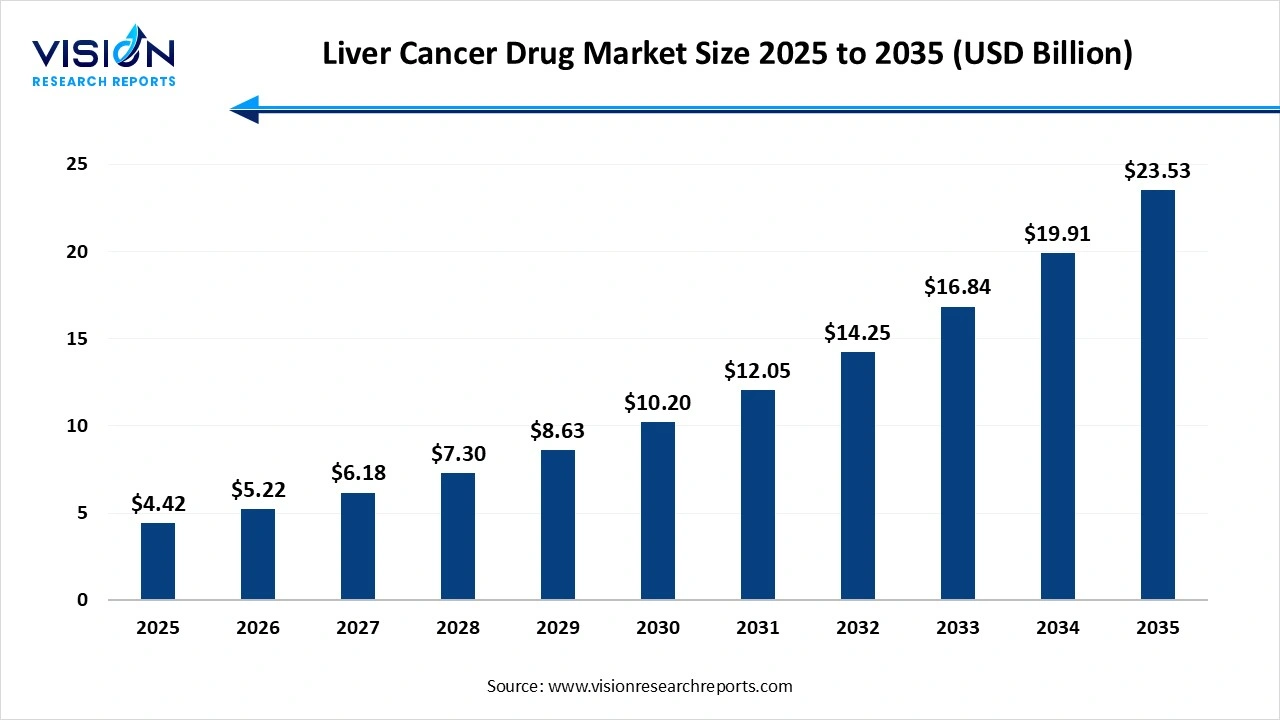
The global liver cancer drug market is experiencing steady growth, driven by the rising prevalence of hepatocellular carcinoma (HCC), increasing adoption of targeted therapies, and expanding research into immuno-oncology. Advancements in diagnostic technologies and improved screening programs are enabling earlier detection, resulting in greater demand for effective treatment options. Targeted therapies and immunotherapies remain at the forefront due to their higher efficacy and favorable safety profiles compared to traditional chemotherapy.
The growth of the liver cancer drug market is propelled by several key factors. Firstly, the escalating global incidence of liver cancer, attributed to factors such as chronic liver diseases and viral hepatitis infections, underscores the urgent need for effective therapeutic interventions. The continuous advancements in research and development by major pharmaceutical companies contribute significantly to the expansion of the market. The emergence of innovative treatment modalities, including targeted therapies and immunotherapies, has reshaped the treatment landscape, providing patients with more tailored and effective options. Strategic collaborations between industry leaders and research institutions further stimulate market growth by fostering a collaborative environment for accelerated drug development. The increasing recognition of immunotherapy as a promising avenue for liver cancer treatment has also played a pivotal role in driving market expansion. As the market continues to address unmet medical needs and capitalize on scientific breakthroughs, these growth factors collectively contribute to the dynamic evolution of the liver cancer drug market.
| Report Coverage | Details |
| Market Size in 2024 | USD 4.42 Billion |
| Revenue Forecast by 2034 | USD 23.53 Billion |
| Growth rate from 2025 to 2034 | CAGR of 18.2% |
| Base Year | 2025 |
| Forecast Period | 2026 to 2035 |
| Regions | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Covered | Bayer AG, Eisai Co., Ltd., Bristol-Myers Squibb Company, Merck & Co., Inc., Roche Holding AG (Genentech), AstraZeneca plc, Novartis AG, Pfizer Inc., Exelixis, Inc., Ipsen Pharma, Eli Lilly and Company, Sanofi S.A. |
North America region dominated the market with the largest market share of 38% in 2024. The region is expected to continue its dominance during the forecast period due to factors such as increasing awareness regarding liver cancer, availability of reimbursement for liver cancer drugs, and strong research and development activities. According to the Centers for Disease Control and Prevention (CDC), cancer is a condition characterized by the uncontrolled growth of cells within the body. When this abnormal cell growth occurs in the liver, it is referred to as liver cancer.
Annually, approximately 11,000 women and 25,000 men are diagnosed with liver cancer in the U.S., with around 19,000 men and 9,000 women losing their lives to this disease. Over the years, the incidence rate of liver cancer in the U.S. experienced an upward trend but has recently started to decline. Despite this decline, liver cancer remains a significant health concern, and its impact on affected individuals and their families is substantial.
In December 2022, the Centers for Medicare & Medicaid Services (CMS) introduced a proposed rule for the Medicare Program in 2024 to enhance health equity and streamline utilization management in Medicare Advantage (MA) plans. The American Society for Radiation Oncology (ASTRO) is in full support of this rule, especially the provisions that require MA plans to follow National Coverage Determinations (NCD), Local Coverage Determinations (LCD), and traditional Medicare regulations, aligning with ASTRO's advocacy efforts. The CMS taking steps to reform MA would result in improved patient care and greater access to essential healthcare services.
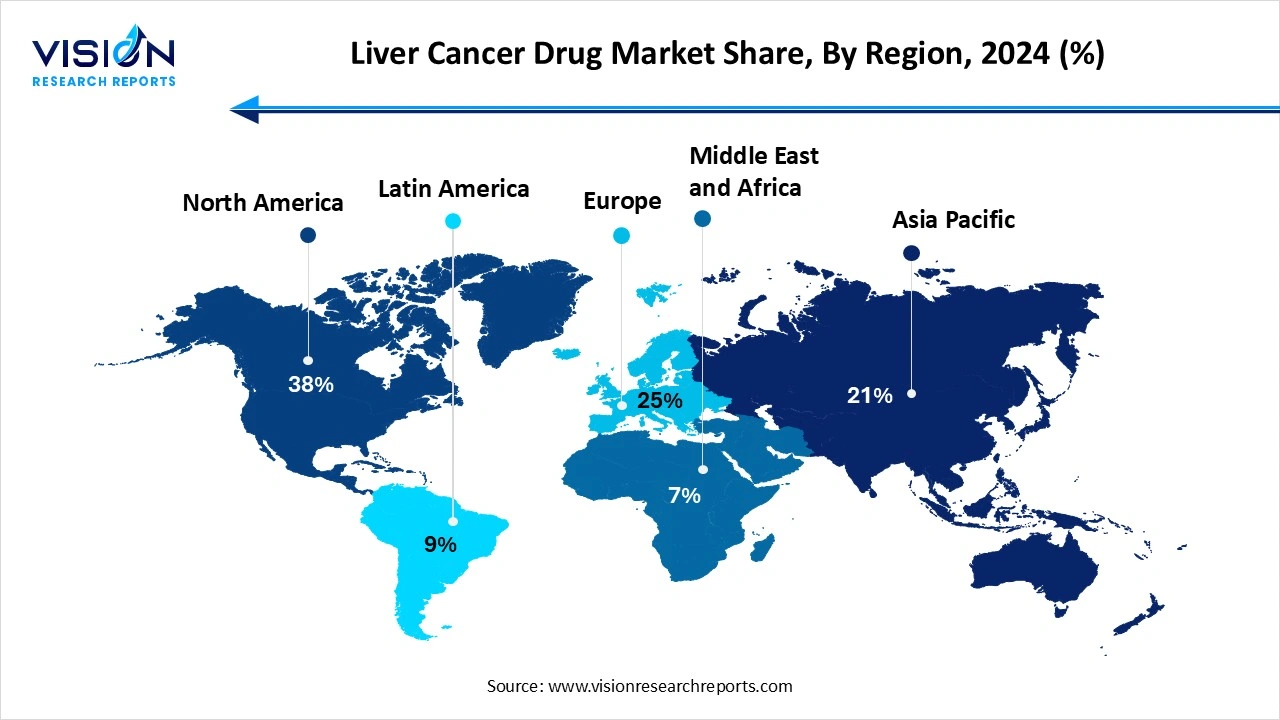 Asia Pacific is expected to grow at the fastest CAGR of 19.7% during the forecast period. This is owing to the high prevalence of liver cancer in the region. As per the NCBI, in Asia, liver cancer is the fifth most common cancer form and the second most common cause of cancer-related deaths. In 2020, approximately 609,596 new liver cancer cases were reported in Asia, accounting for around 72.5% of the global incidence, with an Age-Standardized Rate (ASR) of 11.6 per 100,000.
Asia Pacific is expected to grow at the fastest CAGR of 19.7% during the forecast period. This is owing to the high prevalence of liver cancer in the region. As per the NCBI, in Asia, liver cancer is the fifth most common cancer form and the second most common cause of cancer-related deaths. In 2020, approximately 609,596 new liver cancer cases were reported in Asia, accounting for around 72.5% of the global incidence, with an Age-Standardized Rate (ASR) of 11.6 per 100,000.
Regarding mortality, there were about 566,269 liver cancer-related deaths in Asia in 2020, representing 72.4% of the total liver cancer deaths worldwide. According to a report by Cancer Australia, liver cancer ranked as the seventh most common cause of cancer-related death in the year 2020. It is projected to maintain its position as the seventh most common cause in 2022. In 2020, a total of 2,192 deaths were attributed to liver cancer in Australia, with 1,468 males and 724 females.
Why Did the Targeted Therapy Segment Dominate the Liver Cancer Drug Market?
In 2024, the targeted therapy segment contributed the largest market share of 54%. This dominance is attributed to the ability of targeted therapies to specifically attack cancer cells while minimizing damage to healthy tissues. These therapies can be used alone or in combination with traditional chemotherapy, which works by destroying rapidly dividing cancer cells throughout the body.
Targeted therapy is often recommended when liver cancer has metastasized either spreading to other organs or when cancer from another site has spread to the liver. Its precision-based mechanism and effectiveness in advanced cases have significantly boosted its adoption in treatment protocols.
The immunotherapy segment poised for the fastest growth in the liver cancer drug market. The immunotherapy segment is projected to grow at the fastest rate in the liver cancer drug market. Immunotherapy works by empowering the body’s immune system to recognize and eliminate cancer cells. A key class within this segment is checkpoint inhibitors, which block proteins that prevent immune cells from attacking cancer.
One such checkpoint inhibitor, atezolizumab, is included under the Pharmaceutical Benefits Scheme (PBS) for the treatment of certain primary liver cancers. Atezolizumab, when used in combination with the targeted therapy drug bevacizumab, is now widely recognized as a first-line treatment option for liver cancer. This combination therapy has shown strong efficacy, further driving the rapid expansion of the immunotherapy segment.
Why Did the Hepatocellular Carcinoma Segment Dominate the Liver Cancer Drug Market?
The hepatocellular carcinoma (HCC) segment dominated the liver cancer drug market share of 39% in 2024 because HCC is the most common form of liver cancer, originating from hepatocytes the primary liver cells. The liver, located under the rib cage on the right side of the abdomen, plays a vital role in digestion, detoxification, and metabolic regulation. HCC primarily affects individuals with underlying chronic liver diseases such as cirrhosis, often caused by hepatitis B or hepatitis C infections. Its high prevalence and strong association with chronic liver conditions have significantly contributed to the dominance of this segment.
The Cholangio Carcinoma Segment Is Expected to Grow at the Fastest Rate. Cholangio Carcinoma (CCA), a rare cancer originating in the bile duct epithelium, accounts for nearly 35% of global gastrointestinal cancer cases. Its incidence is particularly high in Southeast and East Asia, with northeastern Thailand reporting the highest burden. CCA is challenging to treat due to limited early detection options and rapid metastasis, resulting in high mortality rates. Conventional chemotherapy options often lead to drug resistance and severe side effects due to off-target drug activity. These clinical challenges and the pressing need for more effective therapies are driving rapid growth in this segment.
Why Did the Hospital Pharmacies Segment Dominate the Market?
The hospital pharmacies dominated the liver cancer drug market share of 46% in 2024. Due to their ability to provide a comprehensive range of advanced medications, including targeted therapies, immunotherapies, and conventional chemotherapies. These drugs are essential for treating hepatocellular carcinoma (HCC) and other forms of liver cancer. Targeted therapies and immunotherapies are typically preferred for advanced or unresectable HCC, while chemotherapy is used selectively when other treatments are ineffective. Additionally, certain therapies such as ramucirumab are prescribed based on specific biomarkers like elevated alpha-fetoprotein (AFP) levels, further reinforcing the role of hospital pharmacies in delivering specialized cancer care.
The Online Pharmacies Segment Is Expected to Grow at the Fastest Rate. The online pharmacies segment is projected to grow rapidly; however, obtaining prescription-based liver cancer medications online without a valid prescription is unsafe and illegal. Purchasing from unverified online platforms increases the risk of receiving counterfeit, substandard, or mislabeled drugs that may be ineffective or harmful. Licensed online pharmacies always require a valid prescription before dispensing such critical medications, ensuring safety and regulatory compliance.
By Therapy
By Type
By Distribution Channel
By Region
Liver Cancer Drug Market
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Therapy Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Liver Cancer Drug Market
5.1. COVID-19 Landscape: Liver Cancer Drug Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Liver Cancer Drug Market, By Therapy
8.1. Liver Cancer Drug Market, by Therapy,
8.1.1 Targeted Therapy
8.1.1.1. Market Revenue and Forecast
8.1.2. Immunotherapy
8.1.2.1. Market Revenue and Forecast
8.1.3. Chemotherapy
8.1.3.1. Market Revenue and Forecast
8.1.4. Others
8.1.4.1. Market Revenue and Forecast
Chapter 9. Global Liver Cancer Drug Market, By Type
9.1. Liver Cancer Drug Market, by Type,
9.1.1. Hepatocellular Carcinoma
9.1.1.1. Market Revenue and Forecast
9.1.2. Cholangio Carcinoma
9.1.2.1. Market Revenue and Forecast
9.1.3. Hepatoblastoma
9.1.3.1. Market Revenue and Forecast
9.1.4. Others
9.1.4.1. Market Revenue and Forecast
Chapter 10. Global Liver Cancer Drug Market, By Distribution Channel
10.1. Liver Cancer Drug Market, by Distribution Channel,
10.1.1. Hospital Pharmacies
10.1.1.1. Market Revenue and Forecast
10.1.2. Retail Pharmacies
10.1.2.1. Market Revenue and Forecast
10.1.3. Online Pharmacies
10.1.3.1. Market Revenue and Forecast
Chapter 11. Global Liver Cancer Drug Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Therapy
11.1.2. Market Revenue and Forecast, by Type
11.1.3. Market Revenue and Forecast, by Distribution Channel
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Therapy
11.1.4.2. Market Revenue and Forecast, by Type
11.1.4.3. Market Revenue and Forecast, by Distribution Channel
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Therapy
11.1.5.2. Market Revenue and Forecast, by Type
11.1.5.3. Market Revenue and Forecast, by Distribution Channel
11.2. Europe
11.2.1. Market Revenue and Forecast, by Therapy
11.2.2. Market Revenue and Forecast, by Type
11.2.3. Market Revenue and Forecast, by Distribution Channel
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Therapy
11.2.4.2. Market Revenue and Forecast, by Type
11.2.4.3. Market Revenue and Forecast, by Distribution Channel
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Therapy
11.2.5.2. Market Revenue and Forecast, by Type
11.2.5.3. Market Revenue and Forecast, by Distribution Channel
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Therapy
11.2.6.2. Market Revenue and Forecast, by Type
11.2.6.3. Market Revenue and Forecast, by Distribution Channel
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Therapy
11.2.7.2. Market Revenue and Forecast, by Type
11.2.7.3. Market Revenue and Forecast, by Distribution Channel
11.3. APAC
11.3.1. Market Revenue and Forecast, by Therapy
11.3.2. Market Revenue and Forecast, by Type
11.3.3. Market Revenue and Forecast, by Distribution Channel
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Therapy
11.3.4.2. Market Revenue and Forecast, by Type
11.3.4.3. Market Revenue and Forecast, by Distribution Channel
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Therapy
11.3.5.2. Market Revenue and Forecast, by Type
11.3.5.3. Market Revenue and Forecast, by Distribution Channel
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Therapy
11.3.6.2. Market Revenue and Forecast, by Type
11.3.6.3. Market Revenue and Forecast, by Distribution Channel
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Therapy
11.3.7.2. Market Revenue and Forecast, by Type
11.3.7.3. Market Revenue and Forecast, by Distribution Channel
11.4. MEA
11.4.1. Market Revenue and Forecast, by Therapy
11.4.2. Market Revenue and Forecast, by Type
11.4.3. Market Revenue and Forecast, by Distribution Channel
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Therapy
11.4.4.2. Market Revenue and Forecast, by Type
11.4.4.3. Market Revenue and Forecast, by Distribution Channel
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Therapy
11.4.5.2. Market Revenue and Forecast, by Type
11.4.5.3. Market Revenue and Forecast, by Distribution Channel
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Therapy
11.4.6.2. Market Revenue and Forecast, by Type
11.4.6.3. Market Revenue and Forecast, by Distribution Channel
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Therapy
11.4.7.2. Market Revenue and Forecast, by Type
11.4.7.3. Market Revenue and Forecast, by Distribution Channel
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Therapy
11.5.2. Market Revenue and Forecast, by Type
11.5.3. Market Revenue and Forecast, by Distribution Channel
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Therapy
11.5.4.2. Market Revenue and Forecast, by Type
11.5.4.3. Market Revenue and Forecast, by Distribution Channel
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Therapy
11.5.5.2. Market Revenue and Forecast, by Type
11.5.5.3. Market Revenue and Forecast, by Distribution Channel
Chapter 12. Company Profiles
12.1. Bayer AG.
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Eisai Co., Ltd.
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Bristol-Myers Squibb Company
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Merck & Co., Inc.
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Roche Holding AG (Genentech)
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. AstraZeneca plc
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Novartis AG.
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Pfizer Inc.
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Eli Lilly and Company.
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Sanofi S.A.
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others