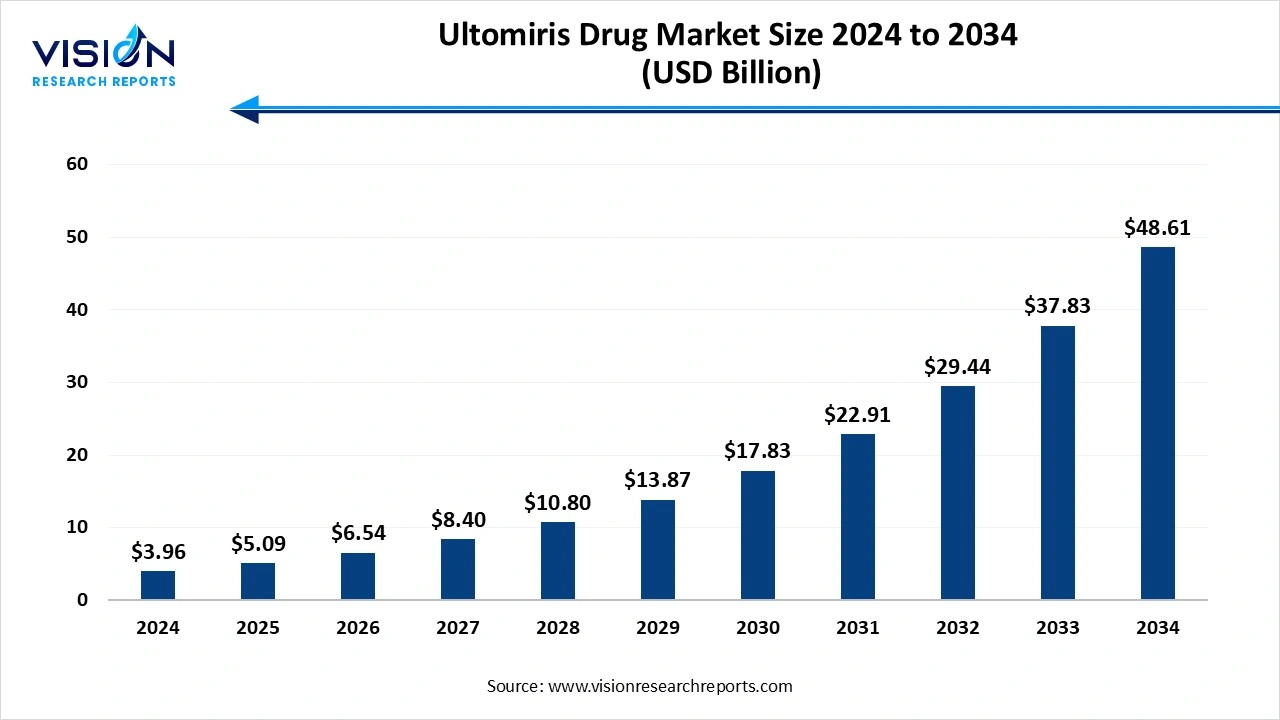
The ultomiris drug market size stood at USD 3.96 billion in 2024 and is estimated to reach USD 5.09 billion in 2025. It is projected to surge past USD 48.61 billion by 2034, registering a robust CAGR of 28.5% from 2025 to 2034. The extended dosing regimen, expanding clinical utility, increasing incidence of complement-mediated disorders and neurological conditions, and advanced diagnostic tools fuel the market growth.
 Key Pointers
Key PointersUltomiris is a prescription-only, long-acting monoclonal antibody drug used to treat several rare autoimmune disorders by inhibiting a key protein in the immune system. The market growth is fueled by Ultomiris offering a significantly longer dosing interval (every 8 weeks) compared to its predecessor, Soliris, which enhances patient convenience and adherence.
This longer duration between treatments improves patient quality of life and reduces the burden associated with frequent infusions. Expanding clinical applications, rising prevalence of rare diseases. Ultomiris has demonstrated robust clinical performance and a manageable safety profile in the treatment of PNH and gMG, driving market growth.
An increase in the diagnosis and treatment of the rare, life-threatening hematologic and autoimmune conditions is indicated. The global incidence and prevalence of PNH and gMG contribute to a growing patient population. Advancements in diagnostic technology enable earlier and more accurate identification of these rare disorders, allowing for quicker treatment initiation with drugs like Ultomiris.
| Report Coverage | Details |
| Market Size in 2024 | USD 3.96 billion |
| Revenue Forecast by 2034 | USD 48.61 billion |
| Growth rate from 2025 to 2034 | CAGR of 28.5% |
| Base Year | 2024 |
| Forecast Period | 2025 to 2034 |
| Regions | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Companies Covered | Roche Holding AG, Novartis AG, Pfizer Inc., Sanofi S.A., Johnson & Johnson, Amgen Inc., Biogen Inc., Regeneron Pharmaceuticals, Inc., and Takeda Pharmaceutical Company Limited. |
The high cost significantly limits patient access, especially in countries with limited resources or restrictive budgets. Payers, including governments and private insurers, are hesitant to approve coverage without strong evidence of the drug's cost-effectiveness compared to other therapies. This creates significant negotiation and reimbursement constraints.
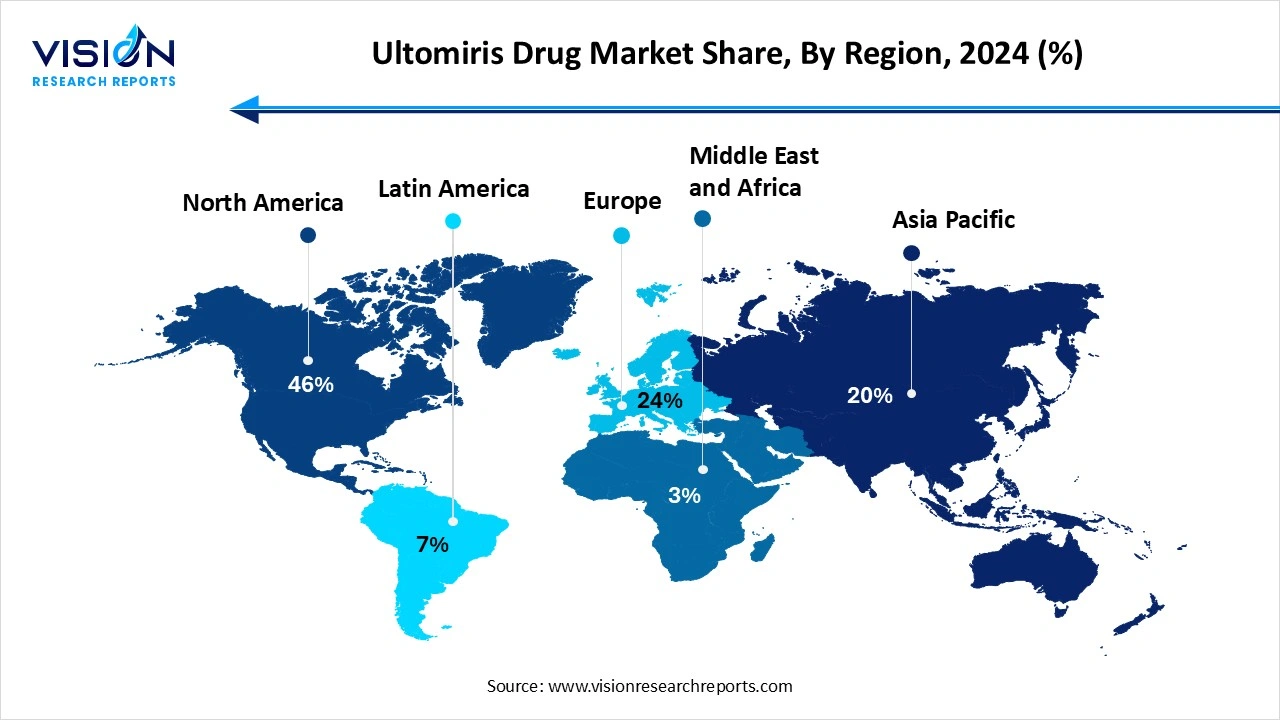
North America held a dominant position in the Ultomiris (ravulizumab) drug market in 2024, capturing over 46% of the total market share. The region has a sophisticated healthcare infrastructure, advanced diagnostic capabilities, robust clinical trial networks, and widespread specialized care centers. Early approvals of ultomeries, especially in the United States, have allowed for quicker uptake compared to other regions. Extensive awareness campaigns and robust clinical trial activity in North America have ensured that both healthcare professionals and patients are well-informed about Ultomiris and its potential to treat rare complement-mediated disorders.
United States Ultomiris Drug Market Trends
The Ultomiris' extended dosing interval offers increased patient convenience and compliance, making it a preferred choice over shorter-acting alternatives like Soliris. The drug has expanded its therapeutic reach beyond its initial uses in paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS).
Asia Pacific expects significant growth in the Ultomiris drug market during the forecast period. The growing awareness and diagnosis of rare diseases, rapid economic growth in countries such as China and India, have led to enhanced healthcare infrastructure. The availability of high-efficacy biologic drugs for rare autoimmune and blood disorders is increasing in the region. Healthcare professionals are becoming increasingly familiar with complement inhibitors like Ultomiris, leading to greater confidence in prescribing it. Increased patient and caregiver awareness about rare conditions and available treatment options contributes to higher adoption rates of Ultomiris.
Why did the Paroxysmal Nocturnal Hemoglobinuria (PNH) Segment Dominate the Ultomiris Drug Market?
The paroxysmal nocturnal hemoglobinuria (PNH) segment dominated the Ultomiris Drug market share 49% in 2024. Ultomiris offered a significantly more convenient dosing schedule than its predecessor, Soliris. This improvement, combined with its strong clinical efficacy and comparable safety profile, drove a widespread patient switch from Soliris to Ultomiris. As the first indication approved for Ultomiris, the well-established PNH patient population fueled the drug's initial market success. This strategic market conversion for a rare disease ultimately cemented PNH's dominant position.
The generalized myasthenia gravis (gMG) segment is the fastest-growing in the Ultomiris drug market during the forecast period. The Ultomiris's strong clinical effectiveness and convenient, long-acting dosing schedule. Regulatory approvals and the expanding utility of the drug for gMG patients have further boosted its market position. This combination of clinical benefits, ease of use, and strategic market expansion has cemented the gMG segment as a key driver of Ultomiris's market success.
How the Adult Segment hold the Largest Share in the Ultomiris Drug Market?
The adult segment held the largest revenue share in the Ultomiris Drug market in 2024. The higher prevalence of its primary indications, PNH and aHUS, in adults. The market share is further solidified by established adult treatment protocols, the drug's extended dosing interval, and the widespread transition of patients from older therapies like Soliris. This preference for a less burdensome, long-acting treatment, supported by a well-developed healthcare infrastructure, reinforces the adult segment's market dominance. While the pediatric market is growing, the adult population's higher disease burden currently ensures its leading position.
The pediatric segment is experiencing the fastest growth in the market during the forecast period. The better standardized pediatric formulations and dosing protocols are promoting wider usage of Ultomiris in children. Increasing awareness of rare diseases in children among caregivers and healthcare professionals, along with improved diagnostic tools, supports earlier detection and treatment initiation. Increasing awareness of rare diseases in children among caregivers and healthcare professionals, along with improved diagnostic tools, supports earlier detection and treatment initiation.
How the Hospital Pharmacies Segment hold the Largest Share in the Ultomiris Drug Market?
The hospital pharmacies segment held the largest revenue share in the Ultomiris drug market in 2024. The specialized care and controlled environments are required for treating rare diseases like PNH and gMG. These pharmacies ensure safe handling, storage, and administration, often managing complex treatment protocols and patient monitoring. Hospital pharmacies possess the necessary expertise and infrastructure to handle and store high-cost biologics like Ultomiris, maintaining quality and ensuring patient safety.
The online pharmacies segment is experiencing the fastest growth in the market during the forecast period. The Ultomiris drug market is experiencing rapid growth driven by increased patient demand for convenience and accessibility. This growth is supported by the rise of home infusion services, which online specialty pharmacies are equipped to handle, and integration with digital health tools for prescription management and patient support. This technological shift enhances patient adherence and reduces hospital visits, positioning online pharmacies as the fastest-growing distribution channel.
By Indication
By End Use
By Distribution Channel
By Regional
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Indication Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Ultomiris Drug Market
5.1. COVID-19 Landscape: Ultomiris Drug Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Ultomiris Drug Market, By Indication
8.1. Ultomiris Drug Market, by Indication
8.1.1 Paroxysmal Nocturnal Hemoglobinuria (PNH)
8.1.1.1. Market Revenue and Forecast
8.1.2. Atypical Hemolytic Uremic Syndrome (aHUS)
8.1.2.1. Market Revenue and Forecast
8.1.3. Generalized Myasthenia Gravis (gMG)
8.1.3.1. Market Revenue and Forecast
8.1.4. Neuromyelitis Optica Spectrum Disorder (NMOSD)
8.1.4.1. Market Revenue and Forecast
Chapter 9. Global Ultomiris Drug Market, By End Use
9.1. Ultomiris Drug Market, by End Use
9.1.1. Adult
9.1.1.1. Market Revenue and Forecast
9.1.2. Pediatric
9.1.2.1. Market Revenue and Forecast
Chapter 10. Global Ultomiris Drug Market, By Distribution Channel
10.1. Ultomiris Drug Market, by Distribution Channel
10.1.1. Hospital Pharmacies
10.1.1.1. Market Revenue and Forecast
10.1.2. Retail Pharmacies
10.1.2.1. Market Revenue and Forecast
10.1.3. Online Pharmacies
10.1.3.1. Market Revenue and Forecast
Chapter 11. Global Ultomiris Drug Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Indication
11.1.2. Market Revenue and Forecast, by End Use
11.1.3. Market Revenue and Forecast, by Distribution Channel
11.1.4. U.S.
11.1.4.1. Market Revenue and Forecast, by Indication
11.1.4.2. Market Revenue and Forecast, by End Use
11.1.4.3. Market Revenue and Forecast, by Distribution Channel
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Forecast, by Indication
11.1.5.2. Market Revenue and Forecast, by End Use
11.1.5.3. Market Revenue and Forecast, by Distribution Channel
11.2. Europe
11.2.1. Market Revenue and Forecast, by Indication
11.2.2. Market Revenue and Forecast, by End Use
11.2.3. Market Revenue and Forecast, by Distribution Channel
11.2.4. UK
11.2.4.1. Market Revenue and Forecast, by Indication
11.2.4.2. Market Revenue and Forecast, by End Use
11.2.4.3. Market Revenue and Forecast, by Distribution Channel
11.2.5. Germany
11.2.5.1. Market Revenue and Forecast, by Indication
11.2.5.2. Market Revenue and Forecast, by End Use
11.2.5.3. Market Revenue and Forecast, by Distribution Channel
11.2.6. France
11.2.6.1. Market Revenue and Forecast, by Indication
11.2.6.2. Market Revenue and Forecast, by End Use
11.2.6.3. Market Revenue and Forecast, by Distribution Channel
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Forecast, by Indication
11.2.7.2. Market Revenue and Forecast, by End Use
11.2.7.3. Market Revenue and Forecast, by Distribution Channel
11.3. APAC
11.3.1. Market Revenue and Forecast, by Indication
11.3.2. Market Revenue and Forecast, by End Use
11.3.3. Market Revenue and Forecast, by Distribution Channel
11.3.4. India
11.3.4.1. Market Revenue and Forecast, by Indication
11.3.4.2. Market Revenue and Forecast, by End Use
11.3.4.3. Market Revenue and Forecast, by Distribution Channel
11.3.5. China
11.3.5.1. Market Revenue and Forecast, by Indication
11.3.5.2. Market Revenue and Forecast, by End Use
11.3.5.3. Market Revenue and Forecast, by Distribution Channel
11.3.6. Japan
11.3.6.1. Market Revenue and Forecast, by Indication
11.3.6.2. Market Revenue and Forecast, by End Use
11.3.6.3. Market Revenue and Forecast, by Distribution Channel
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Forecast, by Indication
11.3.7.2. Market Revenue and Forecast, by End Use
11.3.7.3. Market Revenue and Forecast, by Distribution Channel
11.4. MEA
11.4.1. Market Revenue and Forecast, by Indication
11.4.2. Market Revenue and Forecast, by End Use
11.4.3. Market Revenue and Forecast, by Distribution Channel
11.4.4. GCC
11.4.4.1. Market Revenue and Forecast, by Indication
11.4.4.2. Market Revenue and Forecast, by End Use
11.4.4.3. Market Revenue and Forecast, by Distribution Channel
11.4.5. North Africa
11.4.5.1. Market Revenue and Forecast, by Indication
11.4.5.2. Market Revenue and Forecast, by End Use
11.4.5.3. Market Revenue and Forecast, by Distribution Channel
11.4.6. South Africa
11.4.6.1. Market Revenue and Forecast, by Indication
11.4.6.2. Market Revenue and Forecast, by End Use
11.4.6.3. Market Revenue and Forecast, by Distribution Channel
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Forecast, by Indication
11.4.7.2. Market Revenue and Forecast, by End Use
11.4.7.3. Market Revenue and Forecast, by Distribution Channel
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Indication
11.5.2. Market Revenue and Forecast, by End Use
11.5.3. Market Revenue and Forecast, by Distribution Channel
11.5.4. Brazil
11.5.4.1. Market Revenue and Forecast, by Indication
11.5.4.2. Market Revenue and Forecast, by End Use
11.5.4.3. Market Revenue and Forecast, by Distribution Channel
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Forecast, by Indication
11.5.5.2. Market Revenue and Forecast, by End Use
11.5.5.3. Market Revenue and Forecast, by Distribution Channel
Chapter 12. Company Profiles
12.1. Roche Holding AG.
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Novartis AG.
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Pfizer Inc.
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Sanofi S.A.
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Johnson & Johnson.
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Amgen Inc.
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Biogen Inc.
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Regeneron Pharmaceuticals, Inc.
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Takeda Pharmaceutical Company Limited.
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others