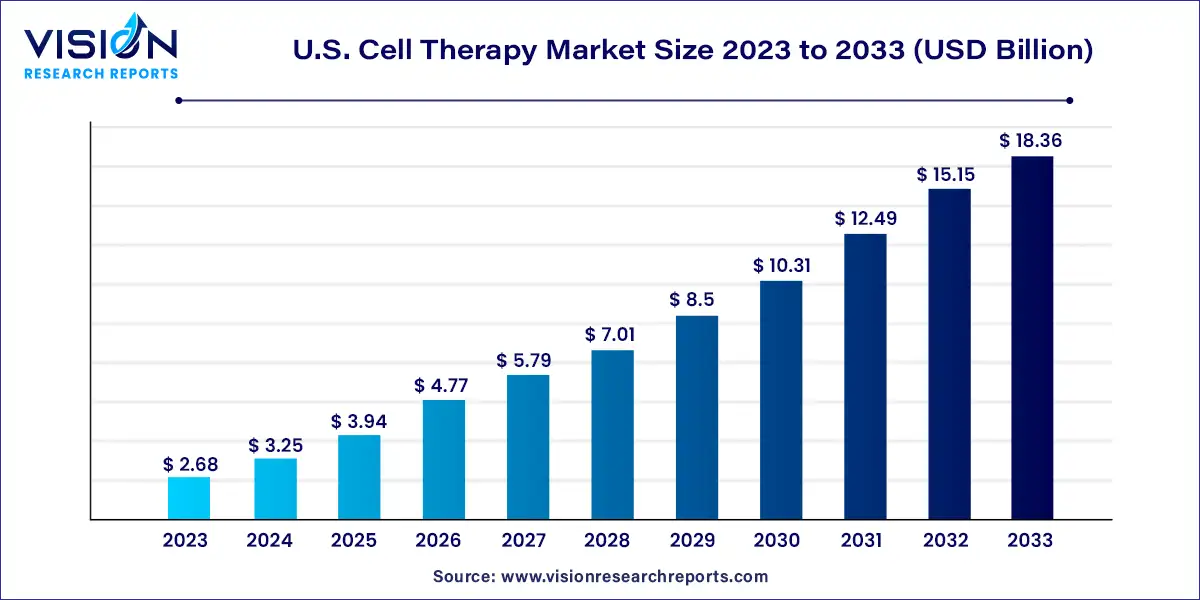
The U.S. Cell Therapy market was estimated at USD 2.68 billion in 2023 and it is expected to surpass around USD 18.36 billion by 2033, poised to grow at a CAGR of 21.22% from 2024 to 2033. The U.S. cell therapy market has witnessed significant growth driven by advancements in biotechnology, increasing investment in research and development, and rising demand for innovative medical treatments.
The growth of the U.S. cell therapy market is driven by an advancement in biotechnology and regenerative medicine have enabled the development of innovative cell-based therapies with the potential to address a wide range of diseases and disorders. Additionally, increasing investment in research and development, coupled with rising demand for effective medical treatments, drives continuous innovation and expansion within the market. Furthermore, favorable regulatory frameworks and reimbursement policies facilitate the commercialization and adoption of cell therapy products, encouraging investment and fostering market growth.
In 2023, the autologous therapies segment asserted its dominance in the market, commanding a substantial 92% share. This growth trajectory is primarily fueled by the personalized nature and broad applicability of these therapies, offering treatment solutions for a diverse array of conditions, including cancer, cardiovascular diseases, neurodegenerative diseases, and orthopedic injuries. Notably, companies are actively pursuing the development of cell therapies tailored for cardiovascular diseases. For instance, BIOCARDIA, INC. is advancing its CardiAMP Cell Therapy, specifically designed to address ischemic heart failure in individuals. This upward trend in autologous therapies is anticipated to sustain and propel demand throughout the forecast period.
Conversely, the allogeneic therapies segment is forecasted to witness the most substantial growth at a robust compound annual growth rate (CAGR) from 2024 to 2033. This surge is attributable to the widespread adoption of allogeneic cell therapies in pioneering innovative treatment approaches. Allogeneic therapies offer distinct advantages over their autologous counterparts, including immediate availability, scalability, and cost-effectiveness. Furthermore, major companies are actively expanding their production capacities for allogeneic cell therapies, a trend poised to further fuel market expansion.
In 2023, the oncology segment firmly held the market, commanding an impressive 93% share. The robust growth of this segment is primarily propelled by the extensive applications of cell therapy within the oncology sector. Various cell therapies, such as CAR T cell therapies, TCR-based therapies, and allogeneic cord blood-based therapies, are deployed in the treatment of diverse cancers, including blood cancer, melanoma, and multiple myeloma. Moreover, companies are intensifying their efforts to advance cell therapies specifically tailored for cancer treatment, further amplifying market expansion prospects.
Conversely, the dermatology segment is poised to experience notable growth at a significant compound annual growth rate (CAGR) from 2024 to 2033. This trajectory is attributed to the continuous advancements in cell therapies aimed at addressing skin disorders. Additionally, the growing emphasis of companies on developing dermatological therapeutics through cell therapy is anticipated to propel segmental growth. For example, in March 2024, SkinCure Oncology unveiled groundbreaking skin cancer treatment technology during the American Academy of Dermatology Annual Meeting in San Diego. This innovation presents a noninvasive treatment option for non-melanoma skin cancer, offering a targeted and efficacious approach with high cure rates.
By Therapy Type
By Therapeutic Area
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on U.S. Cell Therapy Market
5.1. COVID-19 Landscape: U.S. Cell Therapy Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. U.S. Cell Therapy Market, By Therapy Type
8.1. U.S. Cell Therapy Market, by Therapy Type, 2024-2033
8.1.1. Allogeneic Therapies
8.1.1.1. Market Revenue and Forecast (2021-2033)
8.1.2. Autologous Therapies
8.1.2.1. Market Revenue and Forecast (2021-2033)
8.1.3. Others
8.1.3.1. Market Revenue and Forecast (2021-2033)
Chapter 9. U.S. Cell Therapy Market, By Therapeutic Area
9.1. U.S. Cell Therapy Market, by Therapeutic Area, 2024-2033
9.1.1. Oncology
9.1.1.1. Market Revenue and Forecast (2021-2033)
9.1.2. Dermatology
9.1.2.1. Market Revenue and Forecast (2021-2033)
9.1.3. Others
9.1.3.1. Market Revenue and Forecast (2021-2033)
Chapter 10. U.S. Cell Therapy Market, Regional Estimates and Trend Forecast
10.1. U.S.
10.1.1. Market Revenue and Forecast, by Therapy Type (2021-2033)
10.1.2. Market Revenue and Forecast, by Therapeutic Area (2021-2033)
Chapter 11. Company Profiles
11.1. Gilead Sciences, Inc.
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Selecta Bioscience
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Bristol-Myers Squibb Company
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. CARGO Therapeutics, Inc.
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. LTE Scientific
11.5. Johnson & Johnson
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Atara Biotherapeutics, Inc
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Nkarta, Inc
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Cellular Biomedicine Group, Inc
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Vertex Pharmaceuticals Incorporated
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. Aurion Biotech
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
 Cross-segment Market Size and Analysis for
Mentioned Segments
Cross-segment Market Size and Analysis for
Mentioned Segments
 Additional Company Profiles (Upto 5 With No Cost)
Additional Company Profiles (Upto 5 With No Cost)
 Additional Countries (Apart From Mentioned Countries)
Additional Countries (Apart From Mentioned Countries)
 Country/Region-specific Report
Country/Region-specific Report
 Go To Market Strategy
Go To Market Strategy
 Region Specific Market Dynamics
Region Specific Market Dynamics Region Level Market Share
Region Level Market Share Import Export Analysis
Import Export Analysis Production Analysis
Production Analysis Others
Others